In vitro Models of Bone Remodelling and Associated Disorders
- PMID: 30364287
- PMCID: PMC6193121
- DOI: 10.3389/fbioe.2018.00134
In vitro Models of Bone Remodelling and Associated Disorders
Abstract
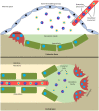
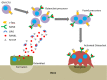
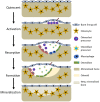
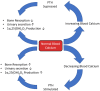
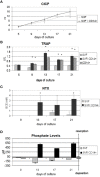
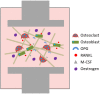
Disruption of bone remodelling by diseases such as osteoporosis results in an imbalance between bone formation by osteoblasts and resorption by osteoclasts. Research into these metabolic bone disorders is primarily performed in vivo; however, in the last decade there has been increased interest in generating in vitro models that can reduce or replace our reliance on animal testing. With recent advances in biomaterials and tissue engineering the feasibility of laboratory-based alternatives is growing; however, to date there are no established in vitro models of bone remodelling. In vivo, remodelling is performed by organised packets of osteoblasts and osteoclasts called bone multicellular units (BMUs). The key determinant of whether osteoclasts form and remodelling occurs is the ratio between RANKL, a cytokine which stimulates osteoclastogenesis, and OPG, its inhibitor. This review initially details the different circumstances, conditions, and factors which have been found to modulate the RANKL:OPG ratio, and fundamental factors to be considered if a robust in vitro model is to be developed. Following this, an examination of what has been achieved thus far in replicating remodelling in vitro using three-dimensional co-cultures is performed, before overviewing how such systems are already being utilised in the study of associated diseases, such as metastatic cancer and dental disorders. Finally, a discussion of the most important considerations to be incorporated going forward is presented. This details the need for the use of cells capable of endogenously producing the required cytokines, application of mechanical stimulation, and the presence of appropriate hormones in order to produce a robust model of bone remodelling.
Keywords: 3D cell culture; bone remodelling; co-culture; in vitro model; osteoblast; osteoclast; osteoporosis; tissue engineering.
Figures


Similar articles
-
Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse.J Bone Miner Res. 2005 Sep;20(9):1659-68. doi: 10.1359/JBMR.050503. Epub 2005 May 2. J Bone Miner Res. 2005. PMID: 16059637
-
Bone remodelling in vitro: Where are we headed?: -A review on the current understanding of physiological bone remodelling and inflammation and the strategies for testing biomaterials in vitro.Bone. 2018 May;110:38-46. doi: 10.1016/j.bone.2018.01.015. Epub 2018 Feb 3. Bone. 2018. PMID: 29355746 Review.
-
Biological aspects of altered bone remodeling in multiple myeloma and possibilities of pharmacological intervention.Dan Med Bull. 2011 May;58(5):B4277. Dan Med Bull. 2011. PMID: 21535989 Review.
-
The differential expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappaB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells.Clin Exp Rheumatol. 2008 Mar-Apr;26(2):295-304. Clin Exp Rheumatol. 2008. PMID: 18565252 Free PMC article.
-
Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway.J Bone Miner Res. 2006 Oct;21(10):1648-56. doi: 10.1359/jbmr.060707. J Bone Miner Res. 2006. PMID: 16995820
Cited by
-
BMP-induced Atoh8 attenuates osteoclastogenesis by suppressing Runx2 transcriptional activity and reducing the Rankl/Opg expression ratio in osteoblasts.Bone Res. 2020 Sep 2;8(1):32. doi: 10.1038/s41413-020-00106-0. eCollection 2020. Bone Res. 2020. PMID: 32923015 Free PMC article.
-
Trabecular bone organoids: a micron-scale 'humanised' prototype designed to study the effects of microgravity and degeneration.NPJ Microgravity. 2021 May 21;7(1):17. doi: 10.1038/s41526-021-00146-8. NPJ Microgravity. 2021. PMID: 34021163 Free PMC article.
-
Recent Developments of Silk-Based Scaffolds for Tissue Engineering and Regenerative Medicine Applications: A Special Focus on the Advancement of 3D Printing.Biomimetics (Basel). 2023 Jan 2;8(1):16. doi: 10.3390/biomimetics8010016. Biomimetics (Basel). 2023. PMID: 36648802 Free PMC article. Review.
-
Peripheral Blood Mononuclear Cells (PBMCs) to Dissect the Underlying Mechanisms of Bone Disease in Chronic Kidney Disease and Rare Renal Diseases.Curr Osteoporos Rep. 2021 Dec;19(6):553-562. doi: 10.1007/s11914-021-00707-6. Epub 2021 Nov 13. Curr Osteoporos Rep. 2021. PMID: 34773213 Review.
-
How Physical Activity across the Lifespan Can Reduce the Impact of Bone Ageing: A Literature Review.Int J Environ Res Public Health. 2020 Mar 13;17(6):1862. doi: 10.3390/ijerph17061862. Int J Environ Res Public Health. 2020. PMID: 32183049 Free PMC article. Review.
References
-
- Andersen T. L., Søe K., Sondergaard T. E., Plesner T., Delaisse J. M. (2010). Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells: research paper. Br. J. Haematol. 148, 551–561. 10.1111/j.1365-2141.2009.07980.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

