Class-B CpG-ODN Formulated With a Nanostructure Induces Type I Interferons-Dependent and CD4+ T Cell-Independent CD8+ T-Cell Response Against Unconjugated Protein Antigen
- PMID: 30364187
- PMCID: PMC6192457
- DOI: 10.3389/fimmu.2018.02319
Class-B CpG-ODN Formulated With a Nanostructure Induces Type I Interferons-Dependent and CD4+ T Cell-Independent CD8+ T-Cell Response Against Unconjugated Protein Antigen
Abstract
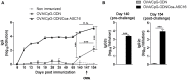
There is a need for new vaccine adjuvant strategies that offer both vigorous antibody and T-cell mediated protection to combat difficult intracellular pathogens and cancer. To this aim, we formulated class-B synthetic oligodeoxynucleotide containing unmethylated cytosine-guanine motifs (CpG-ODN) with a nanostructure (Coa-ASC16 or coagel) formed by self-assembly of 6-0-ascorbyl palmitate ester. Our previous results demonstrated that mice immunized with ovalbumin (OVA) and CpG-ODN formulated with Coa-ASC16 (OVA/CpG-ODN/Coa-ASC16) elicited strong antibodies (IgG1 and IgG2a) and Th1/Th17 cellular responses without toxic systemic effects. These responses were superior to those induced by a solution of OVA with CpG-ODN or OVA/CpG-ODN formulated with aluminum salts. In this study, we investigated the capacity of this adjuvant strategy (CpG-ODN/Coa-ASC16) to elicit CD8+ T-cell response and some of the underlying cellular and molecular mechanisms involved in adaptive response. We also analyzed whether this adjuvant strategy allows a switch from an immunization scheme of three-doses to one of single-dose. Our results demonstrated that vaccination with OVA/CpG-ODN/Coa-ASC16 elicited an antigen-specific long-lasting humoral response and importantly-high quality CD8+ T-cell immunity with a single-dose immunization. Moreover, Coa-ASC16 promoted co-uptake of OVA and CpG-ODN by dendritic cells. The CD8+ T-cell response induced by OVA/CpG-ODN/Coa-ASC16 was dependent of type I interferons and independent of CD4+ T-cells, and showed polyfunctionality and efficiency against an intracellular pathogen. Furthermore, the cellular and humoral responses elicited by the nanostructured formulation were IL-6-independent. This system provides a simple and inexpensive adjuvant strategy with great potential for future rationally designed vaccines.
Keywords: CD8+ T-cell response; CpG-ODN; adjuvant; ascorbyl palmitate ester; nanostructure; type I interferons; vaccine.
Figures


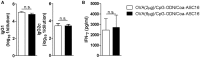



Similar articles
-
Adjuvant activity of CpG-ODN formulated as a liquid crystal.Biomaterials. 2014 Mar;35(8):2529-42. doi: 10.1016/j.biomaterials.2013.12.002. Epub 2013 Dec 30. Biomaterials. 2014. PMID: 24382332
-
Polymeric antigen BLSOmp31 formulated with class B CpG-ODN in a nanostructure (BLSOmp31/CpG-ODN/Coa-ASC16) administered by parenteral or mucosal routes confers protection against Brucella ovis in Balb/c mice.Res Vet Sci. 2021 Mar;135:217-227. doi: 10.1016/j.rvsc.2021.02.011. Epub 2021 Feb 17. Res Vet Sci. 2021. PMID: 33631456
-
Corrigendum to "CpG-ODN formulated with a nanostructure as adjuvant for anticrotalic serum production. Studies in mice" [Toxicon 215 (2022) 28-36].Toxicon. 2022 Sep;216:114. doi: 10.1016/j.toxicon.2022.07.002. Epub 2022 Jul 13. Toxicon. 2022. PMID: 35841861
-
A liquid crystal of ascorbyl palmitate, used as vaccine platform, provides sustained release of antigen and has intrinsic pro-inflammatory and adjuvant activities which are dependent on MyD88 adaptor protein.J Control Release. 2015 Sep 28;214:12-22. doi: 10.1016/j.jconrel.2015.07.008. Epub 2015 Jul 15. J Control Release. 2015. PMID: 26188153
-
CpG-Based Nanovaccines for Cancer Immunotherapy.Int J Nanomedicine. 2021 Aug 5;16:5281-5299. doi: 10.2147/IJN.S317626. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34385817 Free PMC article. Review.
Cited by
-
Difluoromethylornithine (DFMO), an Inhibitor of Polyamine Biosynthesis, and Antioxidant N-Acetylcysteine Potentiate Immune Response in Mice to the Recombinant Hepatitis C Virus NS5B Protein.Int J Mol Sci. 2021 Jun 26;22(13):6892. doi: 10.3390/ijms22136892. Int J Mol Sci. 2021. PMID: 34206987 Free PMC article.
-
Development of a broadly active influenza intranasal vaccine adjuvanted with self-assembled particles composed of mastoparan-7 and CpG.Front Immunol. 2023 Mar 24;14:1103765. doi: 10.3389/fimmu.2023.1103765. eCollection 2023. Front Immunol. 2023. PMID: 37033992 Free PMC article.
-
Research progress of nanovaccine in anti-tumor immunotherapy.Front Oncol. 2023 Aug 24;13:1211262. doi: 10.3389/fonc.2023.1211262. eCollection 2023. Front Oncol. 2023. PMID: 37692854 Free PMC article. Review.
-
A novel CpG ODN compound adjuvant enhances immune response to spike subunit vaccines of porcine epidemic diarrhea virus.Front Immunol. 2024 Jan 23;15:1336239. doi: 10.3389/fimmu.2024.1336239. eCollection 2024. Front Immunol. 2024. PMID: 38322258 Free PMC article.
-
A Vaccine Based on Kunitz-Type Molecule Confers Protection Against Fasciola hepatica Challenge by Inducing IFN-γ and Antibody Immune Responses Through IL-17A Production.Front Immunol. 2020 Oct 20;11:2087. doi: 10.3389/fimmu.2020.02087. eCollection 2020. Front Immunol. 2020. PMID: 33193292 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

