Deletion of TMEM268 inhibits growth of gastric cancer cells by downregulating the ITGB4 signaling pathway
- PMID: 30361615
- PMCID: PMC6748091
- DOI: 10.1038/s41418-018-0223-3
Deletion of TMEM268 inhibits growth of gastric cancer cells by downregulating the ITGB4 signaling pathway
Abstract
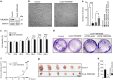
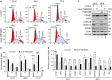
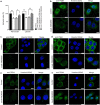
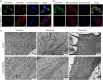
Transmembrane protein 268 (TMEM268) encodes a novel human protein of previously unknown function. This study analyzed the biological activities and molecular mechanisms of TMEM268 in vivo and in vitro. We found that TMEM268 deletion decreases cell viability, proliferation, and cell adhesion as well as causing S-phase cell cycle arrest and disrupts cytoskeleton remolding. Xenograft tumor mouse model studies showed that TMEM268 deletion inhibits the tumorigenesis of BGC823 gastric cancer cells. In addition, TMEM268-deleted BGC823 cells failed to colonize the lungs after intravenous injection and to form metastatic engraftment in the peritoneum. Molecular mechanism studies showed a C-terminal interaction between TMEM268 and integrin subunit β4 (ITGB4). TMEM268 knockout promotes ITGB4 ubiquitin-mediated degradation, increasing the instability of ITGB4 and filamin A (FLNA). The reduced ITGB4 protein levels result in the disassociation of the ITGB4/PLEC complex and cytoskeleton remodeling. This study for the first time demonstrates that TMEM268 plays a positive role in the regulation of ITGB4 homeostasis. The above results may provide a new perspective that targeting the TMEM268/ITGB4 signaling axis for the treatment of gastric cancer, which deserves further investigation in the future.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Deletion of Tmem268 in mice suppresses anti-infectious immune responses by downregulating CD11b signaling.EMBO Rep. 2024 Jun;25(6):2550-2570. doi: 10.1038/s44319-024-00141-6. Epub 2024 May 10. EMBO Rep. 2024. PMID: 38730209 Free PMC article.
-
TCN1 Deficiency Inhibits the Malignancy of Colorectal Cancer Cells by Regulating the ITGB4 Pathway.Gut Liver. 2023 May 15;17(3):412-429. doi: 10.5009/gnl210494. Epub 2022 Jun 10. Gut Liver. 2023. PMID: 35686504 Free PMC article.
-
Metallopanstimulin-1 regulates invasion and migration of gastric cancer cells partially through integrin β4.Carcinogenesis. 2013 Dec;34(12):2851-60. doi: 10.1093/carcin/bgt226. Epub 2013 Jun 26. Carcinogenesis. 2013. PMID: 23803695
-
Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin β4/FAK/SOX2/HIF-1α signaling pathway in gastric cancer.Oncogene. 2018 Feb 8;37(6):744-755. doi: 10.1038/onc.2017.363. Epub 2017 Oct 23. Oncogene. 2018. PMID: 29059156
-
The mechanism of ITGB4 in tumor migration and invasion.Front Oncol. 2024 Aug 7;14:1421902. doi: 10.3389/fonc.2024.1421902. eCollection 2024. Front Oncol. 2024. PMID: 39169946 Free PMC article. Review.
Cited by
-
METTL14-mediated N6-methyladenosine modification of ITGB4 mRNA inhibits metastasis of clear cell renal cell carcinoma.Cell Commun Signal. 2022 Mar 19;20(1):36. doi: 10.1186/s12964-022-00831-5. Cell Commun Signal. 2022. PMID: 35305660 Free PMC article.
-
A Pan-Cancer Analysis of the Oncogenic Role of Integrin Beta4 (ITGB4) in Human Tumors.Int J Gen Med. 2021 Dec 11;14:9629-9645. doi: 10.2147/IJGM.S341076. eCollection 2021. Int J Gen Med. 2021. PMID: 34924769 Free PMC article.
-
ITGB4 as a novel serum diagnosis biomarker and potential therapeutic target for colorectal cancer.Cancer Med. 2021 Oct;10(19):6823-6834. doi: 10.1002/cam4.4216. Epub 2021 Aug 20. Cancer Med. 2021. PMID: 34414684 Free PMC article.
-
Immune Infiltration Subtypes Characterization and Identification of Prognosis-Related lncRNAs in Adenocarcinoma of the Esophagogastric Junction.Front Immunol. 2021 May 28;12:651056. doi: 10.3389/fimmu.2021.651056. eCollection 2021. Front Immunol. 2021. PMID: 34122409 Free PMC article.
-
Reciprocal regulation of integrin β4 and KLF4 promotes gliomagenesis through maintaining cancer stem cell traits.J Exp Clin Cancer Res. 2019 Jan 18;38(1):23. doi: 10.1186/s13046-019-1034-1. J Exp Clin Cancer Res. 2019. PMID: 30658712 Free PMC article.
References
-
- Ishizuka Y, Koshinaga T, Hirano T, Nagasaki-Maeoka E, Watanabe Y, Hoshi R, et al. NRP1 knockdown promotes the migration and invasion of human neuroblastoma-derived SK‑N‑AS cells via the activation of β1 integrin expression. Int J Oncol. 2018;53:159–166.2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

