Pre-Ribosomal RNA Processing in Human Cells: From Mechanisms to Congenital Diseases
- PMID: 30356013
- PMCID: PMC6315592
- DOI: 10.3390/biom8040123
Pre-Ribosomal RNA Processing in Human Cells: From Mechanisms to Congenital Diseases
Abstract
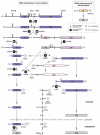
Ribosomal RNAs, the most abundant cellular RNA species, have evolved as the structural scaffold and the catalytic center of protein synthesis in every living organism. In eukaryotes, they are produced from a long primary transcript through an intricate sequence of processing steps that include RNA cleavage and folding and nucleotide modification. The mechanisms underlying this process in human cells have long been investigated, but technological advances have accelerated their study in the past decade. In addition, the association of congenital diseases to defects in ribosome synthesis has highlighted the central place of ribosomal RNA maturation in cell physiology regulation and broadened the interest in these mechanisms. Here, we give an overview of the current knowledge of pre-ribosomal RNA processing in human cells in light of recent progress and discuss how dysfunction of this pathway may contribute to the physiopathology of congenital diseases.
Keywords: Diamond–Blackfan anemia; RNA processing; endonucleases; exonucleases; ribosomal RNAs (rRNAs); ribosomal stress; ribosomopathies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Mutation of ribosomal protein RPS24 in Diamond-Blackfan anemia results in a ribosome biogenesis disorder.Hum Mol Genet. 2008 May 1;17(9):1253-63. doi: 10.1093/hmg/ddn015. Epub 2008 Jan 29. Hum Mol Genet. 2008. PMID: 18230666
-
Domain III of Saccharomyces cerevisiae 25 S ribosomal RNA: its role in binding of ribosomal protein L25 and 60 S subunit formation.J Mol Biol. 2000 Feb 11;296(1):7-17. doi: 10.1006/jmbi.1999.3432. J Mol Biol. 2000. PMID: 10656814
-
[About the ribosomal biogenesis in human].Med Sci (Paris). 2015 Jun-Jul;31(6-7):622-8. doi: 10.1051/medsci/20153106015. Epub 2015 Jul 7. Med Sci (Paris). 2015. PMID: 26152166 Review. French.
-
Probing the mechanisms underlying human diseases in making ribosomes.Biochem Soc Trans. 2016 Aug 15;44(4):1035-44. doi: 10.1042/BST20160064. Biochem Soc Trans. 2016. PMID: 27528749 Free PMC article. Review.
-
[Diamond-Blackfan anemia reveals the dark side of ribosome biogenesis].Med Sci (Paris). 2009 Jan;25(1):69-76. doi: 10.1051/medsci/200925169. Med Sci (Paris). 2009. PMID: 19154697 Review. French.
Cited by
-
Developmental Programming: Sheep Granulosa and Theca Cell-Specific Transcriptional Regulation by Prenatal Testosterone.Endocrinology. 2020 Aug 1;161(8):bqaa094. doi: 10.1210/endocr/bqaa094. Endocrinology. 2020. PMID: 32516392 Free PMC article.
-
TRPV1 Activation Promotes β-arrestin2 Interaction with the Ribosomal Biogenesis Machinery in the Nucleolus:Implications for p53 Regulation and Neurite Outgrowth.Int J Mol Sci. 2021 Feb 25;22(5):2280. doi: 10.3390/ijms22052280. Int J Mol Sci. 2021. PMID: 33668926 Free PMC article.
-
RPL13 Variants Cause Spondyloepimetaphyseal Dysplasia with Severe Short Stature.Am J Hum Genet. 2019 Nov 7;105(5):1040-1047. doi: 10.1016/j.ajhg.2019.09.024. Epub 2019 Oct 17. Am J Hum Genet. 2019. PMID: 31630789 Free PMC article.
-
BCCIP is required for nucleolar recruitment of eIF6 and 12S pre-rRNA production during 60S ribosome biogenesis.Nucleic Acids Res. 2020 Dec 16;48(22):12817-12832. doi: 10.1093/nar/gkaa1114. Nucleic Acids Res. 2020. PMID: 33245766 Free PMC article.
-
CRISPR screening uncovers nucleolar RPL22 as a heterochromatin destabilizer and senescence driver.Nucleic Acids Res. 2024 Oct 28;52(19):11481-11499. doi: 10.1093/nar/gkae740. Nucleic Acids Res. 2024. PMID: 39258545 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

