Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination
- PMID: 30355641
- PMCID: PMC6484683
- DOI: 10.1136/thoraxjnl-2018-211767
Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination
Abstract
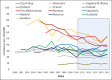
Background: Pneumococcal conjugate vaccines (PCVs) have the potential to prevent pneumococcal disease through direct and indirect protection. This multicentre European study estimated the indirect effects of 5-year childhood PCV10 and/or PCV13 programmes on invasive pneumococcal disease (IPD) in older adults across 13 sites in 10 European countries, to support decision-making on pneumococcal vaccination policies.
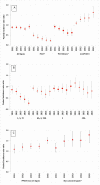
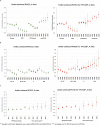
Methods: For each site we calculated IPD incidence rate ratios (IRR) in people aged ≥65 years by serotype for each PCV10/13 year (2011-2015) compared with 2009 (pre-PCV10/13). We calculated pooled IRR and 95% CI using random-effects meta-analysis and PCV10/13 effect as (1 - IRR)*100.
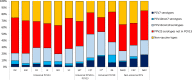
Results: After five PCV10/13 years, the incidence of IPD caused by all types, PCV7 and additional PCV13 serotypes declined 9% (95% CI -4% to 19%), 77% (95% CI 67% to 84%) and 38% (95% CI 19% to 53%), respectively, while the incidence of non-PCV13 serotypes increased 63% (95% CI 39% to 91%). The incidence of serotypes included in PCV13 and not in PCV10 decreased 37% (95% CI 22% to 50%) in six PCV13 sites and increased by 50% (95% CI -8% to 146%) in the four sites using PCV10 (alone or with PCV13). In 2015, PCV13 serotypes represented 20-29% and 32-53% of IPD cases in PCV13 and PCV10 sites, respectively.
Conclusion: Overall IPD incidence in older adults decreased moderately after five childhood PCV10/13 years in 13 European sites. Large declines in PCV10/13 serotype IPD, due to the indirect effect of childhood vaccination, were countered by increases in non-PCV13 IPD, but these declines varied according to the childhood vaccine used. Decision-making on pneumococcal vaccination for older adults must consider the indirect effects of childhood PCV programmes. Sustained monitoring of IPD epidemiology is imperative.
Keywords: bacterial infection; clinical epidemiology.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The following authors report funding for research projects, travels or consultancy outside the submitted work: H-CS (project sponsored by Pfizer); ZH (travel grant from Pfizer), SNL (research including GSK, Pfizer, Sanofi Pasteur on behalf of St George’s University of London and Public Health England (PHE)); NKF (employed by PHE Respiratory and Vaccine Preventable Bacteria Reference Unit and PHE Immunisation that provided serotype surveillance reports to Affinivax, Pfizer and GSK); HR-K and JJ (employed by the National Institute for Health and Welfare that received research funding from GSK for the conduct of a trial of PCV10); AvdE (Pfizer grant for an investigator initiated project, consultancy fees from GSK, participation in the Pfizer Scientific Advisory board); CM-A (fees from GSK and grants from Pfizer); EV (Pfizer grants and personal fees); MC (Pfizer grants and personal fees); HH (research funding from Pfizer and Astellas).
Figures

Similar articles
-
Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: an observational multicentre study.Lancet Respir Med. 2017 Aug;5(8):648-656. doi: 10.1016/S2213-2600(17)30110-8. Epub 2017 Mar 27. Lancet Respir Med. 2017. PMID: 28359798
-
Long-term Impact of a "3 + 0" Schedule for 7- and 13-Valent Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Australia, 2002-2014.Clin Infect Dis. 2017 Jan 15;64(2):175-183. doi: 10.1093/cid/ciw720. Epub 2016 Oct 21. Clin Infect Dis. 2017. PMID: 27986682
-
Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study.Vaccine. 2022 Jun 23;40(29):3963-3974. doi: 10.1016/j.vaccine.2022.05.011. Epub 2022 May 28. Vaccine. 2022. PMID: 35637067
-
Pneumococcal serotype evolution in Western Europe.BMC Infect Dis. 2015 Oct 14;15:419. doi: 10.1186/s12879-015-1147-x. BMC Infect Dis. 2015. PMID: 26468008 Free PMC article. Review.
-
Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis.PLoS One. 2017 May 9;12(5):e0177113. doi: 10.1371/journal.pone.0177113. eCollection 2017. PLoS One. 2017. PMID: 28486544 Free PMC article. Review.
Cited by
-
High prevalence of multiple serotypes of pneumococci in patients with pneumonia and their associated risk factors.Thorax. 2022 Apr 26;77(11):1121-30. doi: 10.1136/thoraxjnl-2021-217979. Online ahead of print. Thorax. 2022. PMID: 35474029 Free PMC article.
-
Estimation of the incidence of hospitalization for non-invasive pneumococcal pneumonia in the Norwegian population aged 50 years and older.Epidemiol Infect. 2022 Apr 4;150:1-21. doi: 10.1017/S0950268822000607. Online ahead of print. Epidemiol Infect. 2022. PMID: 35373724 Free PMC article.
-
Recommendations and Health Technology Assessment (HTA) landscape evaluation for pediatric pneumococcal conjugate vaccines (PCV) in Europe: A systematic literature review.Hum Vaccin Immunother. 2022 Nov 30;18(5):2060017. doi: 10.1080/21645515.2022.2060017. Epub 2022 Apr 19. Hum Vaccin Immunother. 2022. PMID: 35438039 Free PMC article.
-
Mathematical modeling of pneumococcal transmission dynamics in response to PCV13 infant vaccination in Germany predicts increasing IPD burden due to serotypes included in next-generation PCVs.PLoS One. 2023 Feb 15;18(2):e0281261. doi: 10.1371/journal.pone.0281261. eCollection 2023. PLoS One. 2023. PMID: 36791091 Free PMC article.
-
Membrane particles evoke a serotype-independent cross-protection against pneumococcal infection that is dependent on the conserved lipoproteins MalX and PrsA.Proc Natl Acad Sci U S A. 2022 Jun 7;119(23):e2122386119. doi: 10.1073/pnas.2122386119. Epub 2022 Jun 1. Proc Natl Acad Sci U S A. 2022. PMID: 35648835 Free PMC article.
