Neoadjuvant ipilimumab (3 mg/kg or 10 mg/kg) and high dose IFN-α2b in locally/regionally advanced melanoma: safety, efficacy and impact on T-cell repertoire
- PMID: 30352626
- PMCID: PMC6199801
- DOI: 10.1186/s40425-018-0428-5
Neoadjuvant ipilimumab (3 mg/kg or 10 mg/kg) and high dose IFN-α2b in locally/regionally advanced melanoma: safety, efficacy and impact on T-cell repertoire
Abstract
Background: Neoadjuvant immunotherapy utilizing novel combinations has the potential to transform the standard of care for locally/regionally advanced melanoma. We hypothesized that neoadjuvant ipilimumab in combination with high dose IFNα2b (HDI) is safe and associated with durable pathologic complete responses (pCR).
Methods: Patients with locally/regionally advanced melanoma were randomized to ipilimumab 3 or 10 mg/kg × 4 doses bracketing definitive surgery, then every 12 weeks × 4. HDI was given concurrently. We evaluated the safety and efficacy of the combination with ipilimumab 3 or 10 mg/kg. The impact on T-cell fraction and clonality were investigated in tumor and blood.
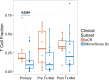
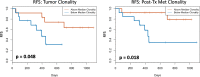
Results: Thirty patients (age 37-76), 15 each at 3 and 10 mg/kg, 18 male and 12 female were treated. Considering immune related adverse events (irAEs) of interest, more grade 3/4 irAEs were seen with ipilimumab 10 mg/kg versus 3 mg/kg (p = 0.042). Among 28 evaluable patients, 11 relapsed, of whom 5 died. Median follow-up for 17 patients who have not relapsed was 32 months. The radiologic preoperative response rate was 36% (95% CI, 21-54); 4 patients at ipilimumab 3 mg/kg and 6 at 10 mg/kg and 2 (at 10 mg/kg) later relapsed. The pCR was 32% (95% CI, 18-51); 5 patients at ipilimumab 3 mg/kg and 4 at 10 mg/kg and one (at 3 mg/kg) had a late relapse. In patients with pCR, T-cell fraction was significantly higher when measured in primary melanoma tumors (p = 0.033). Higher tumor T-cell clonality in primary tumor and more so following neoadjuvant therapy was significantly associated with improved relapse free survival.
Conclusions: Neoadjuvant ipilimumab-HDI was relatively safe and exhibited promising tumor response rates with an associated measurable impact on T-cell fraction and clonality. Most pCRs were durable supporting the value of pCR as a primary endpoint in neoadjuvant immunotherapy trials.
Trial registration: ClinicalTrials.gov, NCT01608594 . Registered 31 May 2012.
Keywords: Anti-CTLA-4; Immunotherapy; Interferon; Ipilimumab; Melanoma.
Conflict of interest statement
Ethics approval and consent to participate
The study was initiated after approval from the institutional review board (IRB) and was conducted in accordance with the Declaration of Helsinki. A University of Pittsburgh IRB approved written informed consent (IRB# PRO12020161) was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
Dr. Tarhini received research grant support Merck, Bristol Myers Squibb and Adaptive Biotechnologies. Dr. Tarhini served as a consultant for Merck, Bristol Myers Squibb, HUYA, Pfizer, Sanofi Genzyme, Novartis, Genentech-Roche, Array Biopharma, NewLink Genetics. John M Kirkwood declared consulting or advisory role to BMS, Merck, Novartis, Roche, Genentech, EMD Serrono, Array Biopharma, Prometheus. Erik C. Yusko and Julie A. Rytlewski are employees or Adaptive Biotechnologies. The rest of the authors declare no potential conflicts of interest relevant to the study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial.Lancet Oncol. 2019 Jul;20(7):948-960. doi: 10.1016/S1470-2045(19)30151-2. Epub 2019 May 31. Lancet Oncol. 2019. PMID: 31160251 Clinical Trial.
-
CTLA-4 blockade and interferon-α induce proinflammatory transcriptional changes in the tumor immune landscape that correlate with pathologic response in melanoma.PLoS One. 2021 Jan 11;16(1):e0245287. doi: 10.1371/journal.pone.0245287. eCollection 2021. PLoS One. 2021. PMID: 33428680 Free PMC article. Clinical Trial.
-
Long term impact of CTLA4 blockade immunotherapy on regulatory and effector immune responses in patients with melanoma.J Transl Med. 2018 Jul 4;16(1):184. doi: 10.1186/s12967-018-1563-y. J Transl Med. 2018. PMID: 29973204 Free PMC article.
-
Neoadjuvant Immunotherapy for Locally Advanced Melanoma.Curr Treat Options Oncol. 2020 Feb 5;21(2):10. doi: 10.1007/s11864-020-0700-z. Curr Treat Options Oncol. 2020. PMID: 32025932 Review.
-
Operable Melanoma: Screening, Prognostication, and Adjuvant and Neoadjuvant Therapy.Am Soc Clin Oncol Educ Book. 2017;37:651-660. doi: 10.1200/EDBK_174930. Am Soc Clin Oncol Educ Book. 2017. PMID: 28561661 Review.
Cited by
-
Predictive factors of neoadjuvant immune checkpoint blockade in melanoma.Hum Vaccin Immunother. 2022 May 31;18(3):1943987. doi: 10.1080/21645515.2021.1943987. Epub 2021 Jul 13. Hum Vaccin Immunother. 2022. PMID: 34254900 Free PMC article. Review.
-
Neoadjuvant treatment for stage III and IV cutaneous melanoma.Cochrane Database Syst Rev. 2023 Jan 17;1(1):CD012974. doi: 10.1002/14651858.CD012974.pub2. Cochrane Database Syst Rev. 2023. PMID: 36648215 Free PMC article. Review.
-
Harnessing cytokines and chemokines for cancer therapy.Nat Rev Clin Oncol. 2022 Apr;19(4):237-253. doi: 10.1038/s41571-021-00588-9. Epub 2022 Jan 7. Nat Rev Clin Oncol. 2022. PMID: 34997230 Review.
-
Neoadjuvant therapy for melanoma: new and evolving concepts.Clin Adv Hematol Oncol. 2022 Jan;20(1):47-55. Clin Adv Hematol Oncol. 2022. PMID: 35060962 Free PMC article. Review.
-
Combining PD-1/PD-L1 blockade with type I interferon in cancer therapy.Front Immunol. 2023 Aug 24;14:1249330. doi: 10.3389/fimmu.2023.1249330. eCollection 2023. Front Immunol. 2023. PMID: 37691915 Free PMC article. Review.
References
-
- Romano E, Scordo M, Dusza SW, Coit DG, Chapman PB. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. Journal of clinical oncology : official journal of the. Proc Am Soc Clin Oncol. 2010;28(18):3042–3047. doi: 10.1200/JCO.2009.26.2063. - DOI - PMC - PubMed
-
- Balch CM, Soong SJ, Smith T, Ross MI, Urist MM, Karakousis CP, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann Surg Oncol. 2001;8(2):101–108. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
