Importance of Promyelocytic Leukema Protein (PML) for Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication
- PMID: 30349510
- PMCID: PMC6186782
- DOI: 10.3389/fmicb.2018.02324
Importance of Promyelocytic Leukema Protein (PML) for Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication
Abstract
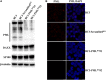
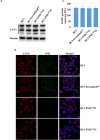
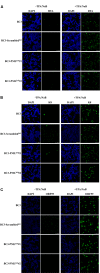
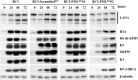
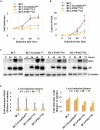
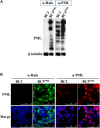
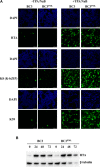
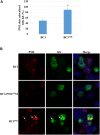
Many DNA virus replication-related proteins are associated with promyelocytic leukemia protein (PML), a component of nuclear domain 10 (ND10), which has been investigated for its potential involvement in viral replication. In the case of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic gene products, K8 (K-bZIP), ORF59, and ORF75 have been shown to colocalize with PML, but its importance in KSHV lytic replication is still unclear. In this study, we analyzed the functional influence of PML on KSHV latency and lytic replication in KSHV-infected primary effusion lymphoma (PEL) cell lines. Stable PML-knockout (BC3-PMLKO) and PML-overexpressing BC3 cells (BC3PML) were successfully generated and the latency and reactivation status were analyzed. The results demonstrated that neither KSHV latency nor the episome copy number was affected in BC3-PMLKO cells. In the reactivation phase, the expression dynamics of KSHV immediate-early or early lytic proteins such as RTA, K9 (vIRF1), K5, K3, ORF59, and K8 (K-bZIP) were comparable between wild-type, control BC3, and BC3-PMLKO cells. Interestingly, KSHV lytic replication, virion production, and expression of late genes were downregulated in BC3-PMLKO cells and upregulated in BC3PML cells, compared to those in control or wild-type BC3 cells. Moreover, exogenous PML increased the size of the PML dots and recruited additional K8 (K-bZIP) to PML-NBs as dots. Therefore, PML would function as a positive regulator for KSHV lytic DNA replication by recruiting KSHV replication factors such as 8 (K-bZIP) or ORF59 to the PML-NBs.
Keywords: Kaposi’s sarcoma-associated herpesvirus (KSHV); ND10; PEL cells; latency; lytic replication; promyelocytic leukema protein (PML).
Figures

Similar articles
-
Kaposi's sarcoma-associated herpesvirus K-bZIP protein is necessary for lytic viral gene expression, DNA replication, and virion production in primary effusion lymphoma cell lines.J Virol. 2009 Jun;83(11):5869-80. doi: 10.1128/JVI.01821-08. Epub 2009 Mar 25. J Virol. 2009. PMID: 19321621 Free PMC article.
-
Full-Length Isoforms of Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen Accumulate in the Cytoplasm of Cells Undergoing the Lytic Cycle of Replication.J Virol. 2017 Nov 30;91(24):e01532-17. doi: 10.1128/JVI.01532-17. Print 2017 Dec 15. J Virol. 2017. PMID: 28978712 Free PMC article.
-
Genome-Wide Identification of Direct RTA Targets Reveals Key Host Factors for Kaposi's Sarcoma-Associated Herpesvirus Lytic Reactivation.J Virol. 2019 Feb 19;93(5):e01978-18. doi: 10.1128/JVI.01978-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541837 Free PMC article.
-
Reactivation and Lytic Replication of Kaposi's Sarcoma-Associated Herpesvirus: An Update.Front Microbiol. 2017 Apr 20;8:613. doi: 10.3389/fmicb.2017.00613. eCollection 2017. Front Microbiol. 2017. PMID: 28473805 Free PMC article. Review.
-
[Replication Machinery of Kaposi's Sarcoma-associated Herpesvirus and Drug Discovery Research].Yakugaku Zasshi. 2019;139(1):69-73. doi: 10.1248/yakushi.18-00164-2. Yakugaku Zasshi. 2019. PMID: 30606932 Review. Japanese.
Cited by
-
Manipulation of Promyelocytic Leukemia Protein Nuclear Bodies by Marek's Disease Virus Encoded US3 Protein Kinase.Microorganisms. 2021 Mar 26;9(4):685. doi: 10.3390/microorganisms9040685. Microorganisms. 2021. PMID: 33810320 Free PMC article.
-
PreS1 Mutations Alter the Large HBsAg Antigenicity of a Hepatitis B Virus Strain Isolated in Bangladesh.Int J Mol Sci. 2020 Jan 15;21(2):546. doi: 10.3390/ijms21020546. Int J Mol Sci. 2020. PMID: 31952213 Free PMC article.
-
The Role of ND10 Nuclear Bodies in Herpesvirus Infection: A Frenemy for the Virus?Viruses. 2021 Feb 3;13(2):239. doi: 10.3390/v13020239. Viruses. 2021. PMID: 33546431 Free PMC article. Review.
-
Regulation of Hepatitis B Virus Replication by Modulating Endoplasmic Reticulum Stress (ER-Stress).Int J Microbiol. 2024 Aug 30;2024:9117453. doi: 10.1155/2024/9117453. eCollection 2024. Int J Microbiol. 2024. PMID: 39246409 Free PMC article.
-
Analysis of the Physicochemical Properties, Replication and Pathophysiology of a Massively Glycosylated Hepatitis B Virus HBsAg Escape Mutant.Viruses. 2021 Nov 22;13(11):2328. doi: 10.3390/v13112328. Viruses. 2021. PMID: 34835134 Free PMC article.
References
-
- Ahn J. H., Brignole E. J., III, Hayward G. S. (1998). Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell. Biol. 18 4899–4913. 10.1128/MCB.18.8.4899 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

