Design, synthesis, and X-ray studies of potent HIV-1 protease inhibitors incorporating aminothiochromane and aminotetrahydronaphthalene carboxamide derivatives as the P2 ligands
- PMID: 30340140
- PMCID: PMC6237192
- DOI: 10.1016/j.ejmech.2018.09.046
Design, synthesis, and X-ray studies of potent HIV-1 protease inhibitors incorporating aminothiochromane and aminotetrahydronaphthalene carboxamide derivatives as the P2 ligands
Abstract
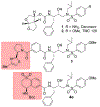
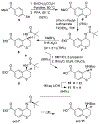
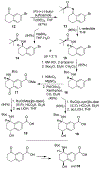
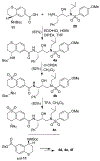
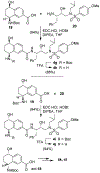
We describe the design, synthesis, and biological evaluation of a series of novel HIV-1 protease inhibitors with carboxamide derivatives as the P2 ligands. We have specifically designed aminothiochromane and aminotetrahydronaphthalene-based carboxamide ligands to promote hydrogen bonding and van der Waals interactions in the active site of HIV-1 protease. Inhibitors 4e and 4j have shown potent enzyme inhibitory and antiviral activity. High resolution X-ray crystal structures of 4d- and 4k-bound HIV-1 protease revealed molecular insights into the ligand-binding site interactions.
Keywords: Design and synthesis; Drug resistance; HIV-1 protease inhibitors; P2 ligand; X-ray crystal structure.
Copyright © 2018 Elsevier Masson SAS. All rights reserved.
Figures
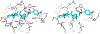
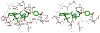
Similar articles
-
Structure-based design, synthesis, X-ray studies, and biological evaluation of novel HIV-1 protease inhibitors containing isophthalamide-derived P2-ligands.Bioorg Med Chem Lett. 2015 Nov 1;25(21):4903-4909. doi: 10.1016/j.bmcl.2015.05.052. Epub 2015 May 30. Bioorg Med Chem Lett. 2015. PMID: 26096678 Free PMC article.
-
Flexible cyclic ethers/polyethers as novel P2-ligands for HIV-1 protease inhibitors: design, synthesis, biological evaluation, and protein-ligand X-ray studies.J Med Chem. 2008 Oct 9;51(19):6021-33. doi: 10.1021/jm8004543. Epub 2008 Sep 11. J Med Chem. 2008. PMID: 18783203 Free PMC article.
-
Design of substituted tetrahydrofuran derivatives for HIV-1 protease inhibitors: synthesis, biological evaluation, and X-ray structural studies.Org Biomol Chem. 2024 Sep 18;22(36):7354-7372. doi: 10.1039/d4ob00506f. Org Biomol Chem. 2024. PMID: 38973505
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
-
Use of molecular dynamics and free energy perturbation calculations in anti-human immunodeficiency virus drug design.Methods Enzymol. 1994;241:370-84. doi: 10.1016/0076-6879(94)41074-7. Methods Enzymol. 1994. PMID: 7854189 Review. No abstract available.
Cited by
-
Design, Synthesis, and Biological Evaluation of Darunavir Analogs as HIV-1 Protease Inhibitors.ACS Bio Med Chem Au. 2024 Sep 19;4(5):242-256. doi: 10.1021/acsbiomedchemau.4c00040. eCollection 2024 Oct 16. ACS Bio Med Chem Au. 2024. PMID: 39431267 Free PMC article.
References
-
- Diffenbach CW, Fauci AS, Thirty Years of HIV and AIDS: Future Challenges and Opportunities, Ann. Intern. Med, 154 (2011) 766–771. - PubMed
-
- Lohse N, Hansen AB, Gerstoft J, Obel N, Improved survival in HIV-infected persons: consequences and perspectives, J. Antimicrob. Chemother, 60 (2007) 461–463. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

