Factors influencing the use of biologic therapy and adoption of treat-to-target recommendations in current European rheumatology practice
- PMID: 30323570
- PMCID: PMC6179241
- DOI: 10.2147/PPA.S170054
Factors influencing the use of biologic therapy and adoption of treat-to-target recommendations in current European rheumatology practice
Abstract
Objective: The aim of this study was to identify factors that influence treatment adjustments and adoption of a treat-to-target (T2T) strategy in patients with rheumatoid arthritis (RA) in European practices.
Methods: Cross-sectional data were drawn from the Adelphi 2014 RA Disease Specific Programme. Treatment patterns and clinical characteristics were investigated in patients treated with biologic disease-modifying antirheumatic drugs (bDMARDs) vs non-bDMARDs. For the T2T analysis, patients were subdivided into two subsets (RA diagnosis <2 or ≥2 years) and compared according to the approach used (no target = no T2T approach; pragmatic = target different from remission; and aspirational = target set as remission).
Results: Data from 2,536 patients were analyzed (mean age: 52.76 years and mean time since RA diagnosis: 6.05 years). Of the 1,438 patients eligible to receive bDMARDs, 55% did not receive them. Initiation of bDMARDs in a bDMARD-naïve patient was prompted by worsening of the disease. In the RA diagnosis <2 years subset, a T2T approach was not adopted in 58% of the patients, whereas 8% and 34% adopted a pragmatic and aspirational approach, respectively. In the RA diagnosis ≥2 years subset, 45%, 19%, and 36% of the patients adopted a no target, pragmatic, and aspirational approach, respectively. Physician satisfaction with RA control was lower in the RA diagnosis <2 years subset than in the RA diagnosis ≥2 years subset (65% vs 77% satisfied, respectively; P<0.0001).
Conclusion: This analysis shows that the use of bDMARDs remains suboptimal and that a T2T strategy is not universally adopted.
Keywords: disease-modifying antirheumatic drugs; rheumatoid arthritis; treat-to-target.
Conflict of interest statement
Disclosure PCT has received fees from AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Merck, Pfizer, Sandoz, Biogen, and UCB Pharma. RA has received fees from AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Merck, Pfizer, Sandoz, Biogen, and UCB Pharma. JJGR has received fees from AbbVie, Biogen, Bristol-Myers Squibb, Hospira, Janssen, Merck, Pfizer, Regeneron, and UCB Pharma. RC has received fees from AbbVie, MSD, Pfizer, Roche, and UCB Pharma. PB has received fees from MSD, Pfizer, Reckitt Benckiser, and Roche. ES, RW, and JP are employees of Adelphi Real World and were contracted by Pfizer to provide data, input into design of data collection, and statistical support for the development of this study. This study was spon sored by Pfizer. Adelphi Real World was contracted by Pfizer to conduct the survey and provide data, input into design of data collection, and statistical support for the development of this study. Preliminary data from this study were presented as posters at the British Society for Rheumatology (BSR) Annual Meeting, April 26–28, 2016, Glasgow, Scotland, and at the Annual Scientific Meeting of the American College of Rheumatology/Association of Rheumatology Health Professionals (ACR/ARHP); November 6–11, 2015; San Francisco, CA, USA. The authors report no other conflicts of interest in this work.
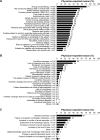
Figures
Similar articles
-
Implementation of the treat-to-target approach and treatment satisfaction in patients with rheumatoid arthritis: perspectives of Chinese rheumatologists.Clin Rheumatol. 2022 Sep;41(9):2659-2668. doi: 10.1007/s10067-022-06187-y. Epub 2022 May 17. Clin Rheumatol. 2022. PMID: 35579773
-
Is treat-to-target really working in rheumatoid arthritis? a longitudinal analysis of a cohort of patients treated in daily practice (RA BIODAM).Ann Rheum Dis. 2020 Apr;79(4):453-459. doi: 10.1136/annrheumdis-2019-216819. Epub 2020 Feb 24. Ann Rheum Dis. 2020. PMID: 32094157
-
Effectiveness and safety of treat-to-target strategy in elderly-onset rheumatoid arthritis: a 3-year prospective observational study.Rheumatology (Oxford). 2021 Sep 1;60(9):4252-4261. doi: 10.1093/rheumatology/keaa922. Rheumatology (Oxford). 2021. PMID: 33410490
-
Treatment strategies are more important than drugs in the management of rheumatoid arthritis.Clin Rheumatol. 2020 Apr;39(4):1363-1368. doi: 10.1007/s10067-020-05001-x. Epub 2020 Feb 22. Clin Rheumatol. 2020. PMID: 32088801 Review.
-
Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation.Health Technol Assess. 2016 Apr;20(35):1-610. doi: 10.3310/hta20350. Health Technol Assess. 2016. PMID: 27140438 Free PMC article. Review.
Cited by
-
Effectiveness of a treat-to-target strategy in patients with moderate to severely active rheumatoid arthritis treated with abatacept.Arthritis Res Ther. 2023 Sep 28;25(1):183. doi: 10.1186/s13075-023-03151-2. Arthritis Res Ther. 2023. PMID: 37759330 Free PMC article. Clinical Trial.
-
Trends in Systemic Glucocorticoid Utilization in the United Kingdom from 1990 to 2019: A Population-Based, Serial Cross-Sectional Analysis.Pragmat Obs Res. 2024 Mar 15;15:53-64. doi: 10.2147/POR.S442959. eCollection 2024. Pragmat Obs Res. 2024. PMID: 38505738 Free PMC article.
-
Physician Adherence to Treat-to-Target and Practice Guidelines in Rheumatoid Arthritis.J Clin Med. 2019 Sep 8;8(9):1416. doi: 10.3390/jcm8091416. J Clin Med. 2019. PMID: 31500394 Free PMC article. Review.
-
Implementation of the treat-to-target approach and treatment satisfaction in patients with rheumatoid arthritis: perspectives of Chinese rheumatologists.Clin Rheumatol. 2022 Sep;41(9):2659-2668. doi: 10.1007/s10067-022-06187-y. Epub 2022 May 17. Clin Rheumatol. 2022. PMID: 35579773
References
-
- Smolen JS, Aletaha D, Mcinnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. - PubMed
-
- Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2016;68(1):1–25. - PubMed
-
- Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. - PubMed

