Stimulation of natural killer cells with rhCD137 ligand enhances tumor-targeting antibody efficacy in gastric cancer
- PMID: 30321186
- PMCID: PMC6188629
- DOI: 10.1371/journal.pone.0204880
Stimulation of natural killer cells with rhCD137 ligand enhances tumor-targeting antibody efficacy in gastric cancer
Abstract
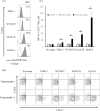
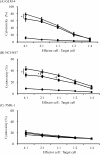
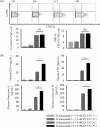
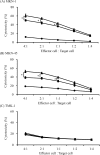
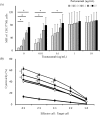
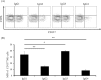
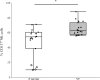
Although many anticancer agents for gastric cancer have been developed, the prognosis for many patients remains poor. Recently, costimulatory immune molecules that reactivate antitumor immune responses by utilizing the host immune system have attracted attention as new therapeutic strategies. CD137 is a costimulatory molecule that reportedly potentiates the antitumor activity of tumor-targeting monoclonal antibodies (mAbs) by enhancing antibody-dependent cellular cytotoxicity. However, it remains unclear whether CD137 stimulates tumor-regulatory activity in gastric cancer. In this study, we investigated the antitumor effects of CD137 stimulation on gastric cancer cells administered tumor-targeting mAbs. Our results showed that human natural killer (NK) cells were activated by expressing CD137 after encountering trastuzumab-coated gastric cancer cells, and that stimulation of activated NK cells in the presence of trastuzumab and recombinant human CD137 ligand (rhCD137L) enhanced cytotoxicity and release of cytokines (IFN-γ, TNF, granzyme A, or granzyme B) as compared with activated NK cells with trastuzumab alone (p < 0.05). By combination treatment with rhCD137L, similar effects were obtained regarding cancer cell cytotoxicity in the presence of cetuximab (p < 0.01). Moreover, we revealed that CD137 expression was dependent upon the affinity between the Fc portion of the antibodies and FcγRIIIa of NK cells based on results indicating that human IgG1 and IgG3 subclasses enhanced CD137 expression (p < 0.001). These results confirmed that FcγRIIIA polymorphisms (158 V/V) enhanced CD137 expression to a greater degree than 158 F polymorphisms (p = 0.014). Our results suggested that CD137 stimulation could promote the effects of tumor-targeting mAbs in gastric cancer, and that further investigation of antibody binding affinity and in vivo activities might improve therapeutic strategies related to the treatment of gastric cancer patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer.Clin Cancer Res. 2017 Feb 1;23(3):707-716. doi: 10.1158/1078-0432.CCR-16-0879. Epub 2016 Aug 5. Clin Cancer Res. 2017. PMID: 27496866 Free PMC article.
-
Anti-CD137 monoclonal antibody enhances trastuzumab-induced, natural killer cell-mediated cytotoxicity against pancreatic cancer cell lines with low human epidermal growth factor-like receptor 2 expression.PLoS One. 2018 Dec 31;13(12):e0200664. doi: 10.1371/journal.pone.0200664. eCollection 2018. PLoS One. 2018. PMID: 30596643 Free PMC article.
-
Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer.J Clin Invest. 2012 Mar;122(3):1066-75. doi: 10.1172/JCI61226. Epub 2012 Feb 13. J Clin Invest. 2012. Retraction in: J Clin Invest. 2019 Jun 03;129(6):2595. doi: 10.1172/JCI129688 PMID: 22326955 Free PMC article. Retracted.
-
Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy.Eur J Immunol. 2016 Mar;46(3):513-22. doi: 10.1002/eji.201445388. Epub 2016 Feb 9. Eur J Immunol. 2016. PMID: 26773716 Review.
-
Harnessing the innate immune system to treat cancer: enhancement of antibody-dependent cellular cytotoxicity with anti-CD137 Ab.Chin Clin Oncol. 2016 Feb;5(1):5. doi: 10.3978/j.issn.2304-3865.2016.02.05. Chin Clin Oncol. 2016. PMID: 26932429 Review. No abstract available.
Cited by
-
CDH17 nanobodies facilitate rapid imaging of gastric cancer and efficient delivery of immunotoxin.Biomater Res. 2022 Nov 26;26(1):64. doi: 10.1186/s40824-022-00312-3. Biomater Res. 2022. PMID: 36435809 Free PMC article.
-
Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment.Nat Rev Drug Discov. 2022 Jun;21(6):440-462. doi: 10.1038/s41573-022-00415-5. Epub 2022 Mar 15. Nat Rev Drug Discov. 2022. PMID: 35292771 Review.
-
The Road Less Taken: Less Appreciated Pathways for Manipulating CD8+ T Cell Exhaustion.Front Immunol. 2022 Jul 6;13:926714. doi: 10.3389/fimmu.2022.926714. eCollection 2022. Front Immunol. 2022. PMID: 35874734 Free PMC article. Review.
-
Boosting Antitumor Response by Costimulatory Strategies Driven to 4-1BB and OX40 T-cell Receptors.Front Cell Dev Biol. 2021 Jun 30;9:692982. doi: 10.3389/fcell.2021.692982. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34277638 Free PMC article. Review.
-
The role of natural killer cells in hepatocellular carcinoma development and treatment: A narrative review.Transl Oncol. 2019 Aug;12(8):1092-1107. doi: 10.1016/j.tranon.2019.04.021. Epub 2019 Jun 6. Transl Oncol. 2019. PMID: 31176993 Free PMC article. Review.
References
-
- Janunger KG, Hafstrom L, Glimelius B. Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg. 2002;168(11):597–608. - PubMed
-
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991–4997. 10.1200/JCO.2006.06.8429 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

