Hepatitis C resistance to NS5A inhibitors: Is it going to be a problem?
- PMID: 30310532
- PMCID: PMC6177567
- DOI: 10.4254/wjh.v10.i9.543
Hepatitis C resistance to NS5A inhibitors: Is it going to be a problem?
Abstract
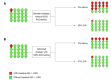
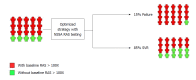
Treatment of hepatitis C virus (HCV) infection has evolved greatly through the recent decade. The availability of direct-acting antiviral agents (DAAs) targeting the functional proteins of HCV has resulted in the introduction of DAA-based combination therapies, providing an optimal rate of treatment success. Among the DAAs, NS5A inhibitors are used in most of the introduced and approved HCV antiviral regimens. Resistance-associated substitutions (RASs) are amino acid substitutions in HCV protein sequences that result in decreased antiviral efficacy of the HCV DAAs. Among the HCV RASs, the NS5A RASs were found to effectively modify and decrease treatment response to NS5A inhibitor-containing regimens. As a baseline predictor of treatment response, NS5A RAS draws attention for pretreatment testing in targeted patient groups. Given NS5A RASs are either naturally-occurring or DAA-selected, the application of NS5A RAS testing can be considered in two settings of NS5A inhibitor-naïve patients and NS5A inhibitor-experienced patients. Less than 5% of NS5A inhibitor-naïve patients harbor naturally-occurring NS5A RAS with high resistance level (> 100X resistance fold-change). In NS5A inhibitor-naïve patients, NS5A RAS testing accompanied by treatment optimization cannot increase treatment response more than 2%-3%, while in NS5A inhibitor-experienced patients, > 75% are found to have NS5A RASs > 100X and NS5A RAS testing in this group of patients seems to be reasonable. This editorial will address the debate on the application of NS5A RAS testing and will discuss if the NS5A RAS testing has any role in clinical management of hepatitis C.
Keywords: Direct-acting antiviral agent; Hepatitis C; NS5A; Resistance; Treatment.
Conflict of interest statement
Conflict-of-interest statement: Both authors declare no potential conflict of interest.
Figures
Similar articles
-
Characteristics of hepatitis C virus resistance in an international cohort after a decade of direct-acting antivirals.JHEP Rep. 2022 Feb 24;4(5):100462. doi: 10.1016/j.jhepr.2022.100462. eCollection 2022 May. JHEP Rep. 2022. PMID: 35434589 Free PMC article.
-
Prevalence of naturally occurring NS5A resistance-associated substitutions in patients infected with hepatitis C virus subtype 1a, 1b, and 3a, co-infected or not with HIV in Brazil.BMC Infect Dis. 2017 Nov 13;17(1):716. doi: 10.1186/s12879-017-2817-7. BMC Infect Dis. 2017. PMID: 29132303 Free PMC article.
-
Patterns of Resistance-Associated Substitutions in Patients With Chronic HCV Infection Following Treatment With Direct-Acting Antivirals.Gastroenterology. 2018 Mar;154(4):976-988.e4. doi: 10.1053/j.gastro.2017.11.007. Epub 2017 Nov 13. Gastroenterology. 2018. PMID: 29146520
-
The Role of RASs /RVs in the Current Management of HCV.Viruses. 2021 Oct 18;13(10):2096. doi: 10.3390/v13102096. Viruses. 2021. PMID: 34696525 Free PMC article. Review.
-
Should NS5A inhibitors serve as the scaffold for all-oral anti-HCV combination therapies?Hepat Med. 2015 Apr 16;7:11-20. doi: 10.2147/HMER.S79584. eCollection 2015. Hepat Med. 2015. PMID: 25926761 Free PMC article. Review.
Cited by
-
Characterization of primary direct-acting antiviral (DAA) drugs resistance mutations in NS5A/NS5B regions of hepatitis C virus with genotype 1a and 1b from patients with chronic hepatitis.Rev Inst Med Trop Sao Paulo. 2022 Sep 30;64:e61. doi: 10.1590/S1678-9946202264061. eCollection 2022. Rev Inst Med Trop Sao Paulo. 2022. PMID: 36197422 Free PMC article.
-
A correlation analysis of the serum hepcidin concentrations and viral loads in HCV-infected patients.Am J Transl Res. 2021 Jun 15;13(6):6297-6304. eCollection 2021. Am J Transl Res. 2021. PMID: 34306369 Free PMC article.
-
Selection dynamics of HCV genotype 3 resistance-associated substitutions under direct-acting antiviral therapy pressure.Braz J Infect Dis. 2022 Nov-Dec;26(6):102717. doi: 10.1016/j.bjid.2022.102717. Epub 2022 Nov 19. Braz J Infect Dis. 2022. PMID: 36410397 Free PMC article.
-
Prevalence of Naturally-Occurring NS5A and NS5B Resistance-Associated Substitutions in Iranian Patients With Chronic Hepatitis C Infection.Front Microbiol. 2021 Jan 28;11:617375. doi: 10.3389/fmicb.2020.617375. eCollection 2020. Front Microbiol. 2021. PMID: 33584581 Free PMC article.
-
High success rates for the use of sofosbuvir/ombitasvir/paritaprevir/ritonavir + ribavirin and sofosbuvir/simeprevir/daclatasvir + ribavirin in retreatment of chronic hepatitis C infection after unsuccessful sofosbuvir/daclatasvir therapy: a real-life experience.Arch Virol. 2020 Jul;165(7):1633-1639. doi: 10.1007/s00705-020-04639-x. Epub 2020 Apr 30. Arch Virol. 2020. Retraction in: Arch Virol. 2024 Feb 13;169(3):45. doi: 10.1007/s00705-024-05990-z PMID: 32356185 Retracted. Clinical Trial.
References
-
- Sharafi H, Nikbin M, Alavian SH, Behnava B, Alavian SM. Efficacy and Safety of Generic Sofosbuvir/Ledipasvir Fixed-Dose Combination in Iranian Patients with Chronic Hepatitis C Virus Infection. Hepat Mon. 2017;17:e12216.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

