In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin's Lymphomas
- PMID: 30309819
- PMCID: PMC6277484
- DOI: 10.1016/j.ymthe.2018.09.009
In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin's Lymphomas
Abstract
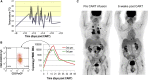
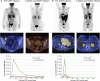
Second-generation (2G) chimeric antigen receptors (CARs) targeting CD19 are highly active against B cell malignancies, but it is unknown whether any of the costimulatory domains incorporated in the CAR have superior activity to others. Because CD28 and 4-1BB signaling activate different pathways, combining them in a single third-generation (3G) CAR may overcome the limitations of each individual costimulatory domain. We designed a clinical trial in which two autologous CD19-specific CAR-transduced T cell products (CD19.CARTs), 2G (with CD28 only) and 3G (CD28 and 4-1BB), were infused simultaneously in 16 patients with relapsed or refractory non-Hodgkin's lymphoma. 3G CD19.CARTs had superior expansion and longer persistence than 2G CD19.CARTs. This difference was most striking in the five patients with low disease burden and few circulating normal B cells, in whom 2G CD19.CARTs had limited expansion and persistence and correspondingly reduced area under the curve. Of the 11 patients with measurable disease, three achieved complete responses and three had partial responses. Cytokine release syndrome occurred in six patients but was mild, and no patient required anti-IL-6 therapy. Hence, 3G CD19.CARTs combining 4-1BB with CD28 produce superior CART expansion and may be of particular value when treating low disease burden in patients whose normal B cells are depleted by prior therapy.
Keywords: CAR-T cells; CD19; chimeric antigen receptor; immunotherapy; second-generation CAR; third-generation CAR.
Copyright © 2018 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Treatment of patients with relapsed or refractory CD19+ lymphoid disease with T lymphocytes transduced by RV-SFG.CD19.CD28.4-1BBzeta retroviral vector: a unicentre phase I/II clinical trial protocol.BMJ Open. 2019 May 19;9(5):e026644. doi: 10.1136/bmjopen-2018-026644. BMJ Open. 2019. PMID: 31110096 Free PMC article. Clinical Trial.
-
Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL.Blood. 2016 Jun 16;127(24):2980-90. doi: 10.1182/blood-2015-12-686725. Epub 2016 Apr 26. Blood. 2016. PMID: 27118452 Free PMC article. Clinical Trial.
-
In Vivo Expansion and Antitumor Activity of Coinfused CD28- and 4-1BB-Engineered CAR-T Cells in Patients with B Cell Leukemia.Mol Ther. 2018 Apr 4;26(4):976-985. doi: 10.1016/j.ymthe.2018.01.022. Epub 2018 Feb 2. Mol Ther. 2018. PMID: 29503204 Free PMC article. Clinical Trial.
-
Chimeric Antigen Receptor T Cell Therapy for Pediatric B-ALL: Narrowing the Gap Between Early and Long-Term Outcomes.Front Immunol. 2020 Aug 11;11:1985. doi: 10.3389/fimmu.2020.01985. eCollection 2020. Front Immunol. 2020. PMID: 32849662 Free PMC article. Review.
-
Chimeric Antigen Receptor T-Cell Therapies for Aggressive B-Cell Lymphomas: Current and Future State of the Art.Am Soc Clin Oncol Educ Book. 2019 Jan;39:446-453. doi: 10.1200/EDBK_238693. Epub 2019 May 17. Am Soc Clin Oncol Educ Book. 2019. PMID: 31099671 Review.
Cited by
-
The antigen-binding moiety in the driver's seat of CARs.Med Res Rev. 2022 Jan;42(1):306-342. doi: 10.1002/med.21818. Epub 2021 May 24. Med Res Rev. 2022. PMID: 34028069 Free PMC article. Review.
-
Decoding CAR T cell phenotype using combinatorial signaling motif libraries and machine learning.Science. 2022 Dec 16;378(6625):1194-1200. doi: 10.1126/science.abq0225. Epub 2022 Dec 8. Science. 2022. PMID: 36480602 Free PMC article.
-
Understanding the Mechanisms of Resistance to CAR T-Cell Therapy in Malignancies.Front Oncol. 2019 Nov 21;9:1237. doi: 10.3389/fonc.2019.01237. eCollection 2019. Front Oncol. 2019. PMID: 31824840 Free PMC article. Review.
-
Enhancing co-stimulation of CAR T cells to improve treatment outcomes in solid cancers.Immunother Adv. 2021 Jul 31;1(1):ltab016. doi: 10.1093/immadv/ltab016. eCollection 2021 Jan. Immunother Adv. 2021. PMID: 35919743 Free PMC article. Review.
-
CD8+ T Cells Expressing an HLA-DR1 Chimeric Antigen Receptor Target Autoimmune CD4+ T Cells in an Antigen-Specific Manner and Inhibit the Development of Autoimmune Arthritis.J Immunol. 2022 Jan 1;208(1):16-26. doi: 10.4049/jimmunol.2100643. Epub 2021 Nov 24. J Immunol. 2022. PMID: 34819392 Free PMC article.
References
-
- Kochenderfer J.N., Wilson W.H., Janik J.E., Dudley M.E., Stetler-Stevenson M., Feldman S.A., Maric I., Raffeld M., Nathan D.A., Lanier B.J. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. - PMC - PubMed
-
- Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

