Enhanced gastrointestinal passive paracellular permeability contributes to the obesity-associated hyperoxaluria
- PMID: 30307745
- PMCID: PMC6383380
- DOI: 10.1152/ajpgi.00266.2018
Enhanced gastrointestinal passive paracellular permeability contributes to the obesity-associated hyperoxaluria
Abstract
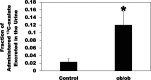
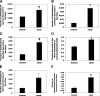
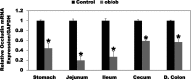
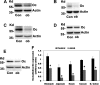
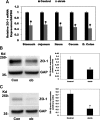
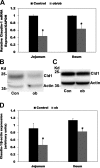
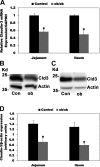
Most kidney stones (KS) are composed of calcium oxalate and small increases in urine oxalate enhance the stone risk. Obesity is a risk factor for KS, and urinary oxalate excretion increases with increased body size. We previously established the obese ob/ob ( ob) mice as a model (3.3-fold higher urine oxalate) to define the pathogenesis of obesity-associated hyperoxaluria (OAH). The purpose of this study was to test the hypothesis that the obesity-associated enhanced small intestinal paracellular permeability contributes to OAH by increasing passive paracellular intestinal oxalate absorption. ob Mice have significantly higher jejunal (1.6-fold) and ileal (1.4-fold) paracellular oxalate absorption ex vivo and significantly higher (5-fold) urine [13C]oxalate following oral gavage with [13C]oxalate, indicating increased intestinal oxalate absorption in vivo. The observation of higher oxalate absorption in vivo compared with ex vivo suggests the possibility of increased paracellular permeability along the entire gut. Indeed, ob mice have significantly higher fractions of the administered sucrose (1.7-fold), lactulose (4.4-fold), and sucralose (3.1-fold) excreted in the urine, reflecting increased gastric, small intestinal, and colonic paracellular permeability, respectively. The ob mice have significantly reduced gastrointestinal occludin, zonula occludens-1, and claudins-1 and -3 mRNA and total protein expression. Proinflammatory cytokines and oxidative stress, which are elevated in obesity, significantly enhanced paracellular intestinal oxalate absorption in vitro and ex vivo. We conclude that obese mice have significantly higher intestinal oxalate absorption and enhanced gastrointestinal paracellular permeability in vivo, which would likely contribute to the pathogenesis of OAH, since there is a transepithelial oxalate concentration gradient to drive paracellular intestinal oxalate absorption. NEW & NOTEWORTHY This study shows that the obese ob/ob mice have significantly increased gastrointestinal paracellular oxalate absorption and remarkably enhanced paracellular permeability along the entire gut in vivo, which are likely mediated by the obesity-associated increased systemic and intestinal inflammation and oxidative stress. A transepithelial oxalate concentration gradient driving gastrointestinal paracellular oxalate absorption exists, and therefore, our novel findings likely contribute to the hyperoxaluria observed in the ob/ob mice and hence to the pathogenesis of obesity-associated hyperoxaluria.
Keywords: gastrointestinal paracellular permeability; hyperoxaluria; inflammation; intestinal oxalate absorption; obesity; oxidative stress; tight junction proteins.
Figures
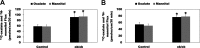

Similar articles
-
Reduced active transcellular intestinal oxalate secretion contributes to the pathogenesis of obesity-associated hyperoxaluria.Kidney Int. 2018 May;93(5):1098-1107. doi: 10.1016/j.kint.2017.11.011. Epub 2018 Feb 1. Kidney Int. 2018. PMID: 29395336 Free PMC article.
-
Jejunal villus absorption and paracellular tight junction permeability are major routes for early intestinal uptake of food-grade TiO2 particles: an in vivo and ex vivo study in mice.Part Fibre Toxicol. 2020 Jun 11;17(1):26. doi: 10.1186/s12989-020-00357-z. Part Fibre Toxicol. 2020. PMID: 32527323 Free PMC article.
-
Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis.Am J Physiol Gastrointest Liver Physiol. 2007 Feb;292(2):G518-25. doi: 10.1152/ajpgi.00024.2006. Epub 2006 Oct 5. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17023554
-
Intestinal adaptations in chronic kidney disease and the influence of gastric bypass surgery.Exp Physiol. 2014 Sep;99(9):1163-7. doi: 10.1113/expphysiol.2014.078782. Epub 2014 Jun 20. Exp Physiol. 2014. PMID: 24951497 Free PMC article. Review.
-
Alterations in intestinal transport of oxalate in disease states.Scanning Microsc. 1995;9(4):1121-6; discussion 1126. Scanning Microsc. 1995. PMID: 8819893 Review.
Cited by
-
Cadmium exposure induces changes in gut microbial composition and metabolic function in long-tailed dwarf hamsters, Cricetulus longicaudatus.Ecol Evol. 2024 Jul 4;14(7):e11682. doi: 10.1002/ece3.11682. eCollection 2024 Jul. Ecol Evol. 2024. PMID: 38966245 Free PMC article.
-
Urinary oxalate as a potential mediator of kidney disease in diabetes mellitus and obesity.Curr Opin Nephrol Hypertens. 2019 Jul;28(4):316-320. doi: 10.1097/MNH.0000000000000515. Curr Opin Nephrol Hypertens. 2019. PMID: 31045662 Free PMC article. Review.
-
Paracellular permeability and tight junction regulation in gut health and disease.Nat Rev Gastroenterol Hepatol. 2023 Jul;20(7):417-432. doi: 10.1038/s41575-023-00766-3. Epub 2023 Apr 25. Nat Rev Gastroenterol Hepatol. 2023. PMID: 37186118 Free PMC article. Review.
-
Oxalate (dys)Metabolism: Person-to-Person Variability, Kidney and Cardiometabolic Toxicity.Genes (Basel). 2023 Aug 29;14(9):1719. doi: 10.3390/genes14091719. Genes (Basel). 2023. PMID: 37761859 Free PMC article. Review.
-
Effects of the Gut Microbiota and Barrier Function on Melatonin Efficacy in Alleviating Liver Injury.Antioxidants (Basel). 2022 Aug 31;11(9):1727. doi: 10.3390/antiox11091727. Antioxidants (Basel). 2022. PMID: 36139801 Free PMC article.
References
-
- Amin R, Asplin J, Jung D, Bashir M, Alshaikh A, Ratakonda S, Sharma S, Jeon S, Granja I, Matern D, Hassan H. Reduced active transcellular intestinal oxalate secretion contributes to the pathogenesis of obesity-associated hyperoxaluria. Kidney Int 93: 1098–1107, 2018. doi:10.1016/j.kint.2017.11.011. - DOI - PMC - PubMed
-
- Arvans D, Jung YC, Antonopoulos D, Koval J, Granja I, Bashir M, Karrar E, Roy-Chowdhury J, Musch M, Asplin J, Chang E, Hassan H. Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J Am Soc Nephrol 28: 876–887, 2017. doi:10.1681/ASN.2016020132. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

