Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage
- PMID: 30307611
- PMCID: PMC6515924
- DOI: 10.1002/cncr.31720
Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage
Abstract
Background: The outcomes of patients with relapsed or refractory (R-R) acute lymphoblastic leukemia (ALL) are poor. Inotuzumab ozogamicin and blinatumomab have single-agent activity in R-R ALL. Their addition to low-intensity chemotherapy may further improve the outcomes of patients with ALL in their first relapse.
Methods: The chemotherapy was lower in intensity than conventional hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone and was called mini-hyperfractionated cyclophosphamide, vincristine, and dexamethasone (or mini-HCVD). Inotuzumab was given on day 3 of each of the first 4 cycles at 1.8 to 1.3 mg/m2 for cycle 1, and this was followed by 1.3 to 1.0 mg/m2 for subsequent cycles. From patient 39 onward, the inotuzumab dose was reduced and fractionated into weekly doses (0.6 and 0.3 mg/m2 during cycle 1 and 0.3 and 0.3 mg/m2 during subsequent cycles), and blinatumomab was administered for up to 4 cycles after inotuzumab therapy.
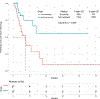
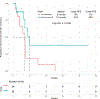
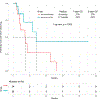
Results: Forty-eight patients with Philadelphia chromosome-negative ALL with a median age of 39 years were treated during their first relapse. Overall, 44 patients (92%) responded, with 35 of them (73%) achieving a complete response. The overall minimal residual disease negativity rate among the responders was 93%. Twenty-four patients (50%) underwent allogeneic stem cell transplantation (ASCT). Veno-occlusive disease of any grade occurred in 5 patients (10%). With a median follow-up of 31 months, the median progression-free survival (PFS) and the median overall survival (OS) were 11 and 25 months, respectively. The 2-year PFS and OS rates were 42% and 54%, respectively. Of the 24 patients (50%) who underwent ASCT, 14 patients were alive at the last follow-up (13 [54%] in remission). Of the remaining 20 responding patients who did not undergo subsequent ASCT, 6 (30%) remained in remission at the last follow-up. According to propensity score matching, the combination of mini-HCVD and inotuzumab with or without blinatumomab conferred better outcomes than intensive salvage chemotherapy or inotuzumab alone.
Conclusions: The combination of inotuzumab and low-intensity mini-HCVD chemotherapy with or without blinatumomab shows encouraging results in patients with ALL in first salvage.
Keywords: acute lymphoblastic leukemia (ALL); first relapse; inotuzumab ozogamicin; salvage.
© 2018 American Cancer Society.
Conflict of interest statement
Figures




Similar articles
-
Salvage Chemoimmunotherapy With Inotuzumab Ozogamicin Combined With Mini-Hyper-CVD for Patients With Relapsed or Refractory Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: A Phase 2 Clinical Trial.JAMA Oncol. 2018 Feb 1;4(2):230-234. doi: 10.1001/jamaoncol.2017.2380. JAMA Oncol. 2018. PMID: 28859185 Free PMC article. Clinical Trial.
-
Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-HCVD) with or without blinatumomab versus standard intensive chemotherapy (HCVAD) as frontline therapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: A propensity score analysis.Cancer. 2019 Aug 1;125(15):2579-2586. doi: 10.1002/cncr.32139. Epub 2019 Apr 15. Cancer. 2019. PMID: 30985931 Free PMC article.
-
Long-term follow-up of salvage therapy using a combination of inotuzumab ozogamicin and mini-hyper-CVD with or without blinatumomab in relapsed/refractory Philadelphia chromosome-negative acute lymphoblastic leukemia.Cancer. 2021 Jun 15;127(12):2025-2038. doi: 10.1002/cncr.33469. Epub 2021 Mar 19. Cancer. 2021. PMID: 33740268
-
Philadelphia chromosome-positive acute lymphoblastic leukemia at first relapse in the era of tyrosine kinase inhibitors.Am J Hematol. 2019 Dec;94(12):1388-1395. doi: 10.1002/ajh.25648. Epub 2019 Oct 21. Am J Hematol. 2019. PMID: 31595534 Review.
-
The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades.J Hematol Oncol. 2023 Mar 16;16(1):22. doi: 10.1186/s13045-023-01409-5. J Hematol Oncol. 2023. PMID: 36927623 Free PMC article. Review.
Cited by
-
Immunotherapy in pediatric acute lymphoblastic leukemia.Cancer Metastasis Rev. 2019 Dec;38(4):595-610. doi: 10.1007/s10555-019-09834-0. Cancer Metastasis Rev. 2019. PMID: 31811553 Free PMC article. Review.
-
Infectious complications during monoclonal antibodies treatments and cell therapies in Acute Lymphoblastic Leukemia.Clin Exp Med. 2023 Oct;23(6):1823-1833. doi: 10.1007/s10238-023-01000-9. Epub 2023 Jan 30. Clin Exp Med. 2023. PMID: 36715833 Free PMC article. Review.
-
Pediatric chemotherapy versus allo-HSCT for adolescent and adult Philadelphia chromosome-negative ALL in first complete remission: a meta-analysis.Ann Hematol. 2023 May;102(5):1131-1140. doi: 10.1007/s00277-023-05160-2. Epub 2023 Mar 22. Ann Hematol. 2023. PMID: 36947212
-
Oncogenic and immunological targets for matched therapy of pediatric blood cancer patients: Dutch iTHER study experience.Hemasphere. 2024 Jul 15;8(7):e122. doi: 10.1002/hem3.122. eCollection 2024 Jul. Hemasphere. 2024. PMID: 39011126 Free PMC article.
-
The Care of the Leukemic Patients in Times of SARS-CoV-2.Curr Oncol Rep. 2021 Aug 3;23(10):114. doi: 10.1007/s11912-021-01111-0. Curr Oncol Rep. 2021. PMID: 34342734 Free PMC article. Review.
References
-
- Jabbour E, O’Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121(15):2517–2528. - PubMed
-
- Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–50. - PubMed

