Predilection of Low Protein C-induced Spontaneous Atherothrombosis for the Right Coronary Sinus in Apolipoprotein E deficient mice
- PMID: 30305662
- PMCID: PMC6180072
- DOI: 10.1038/s41598-018-32584-y
Predilection of Low Protein C-induced Spontaneous Atherothrombosis for the Right Coronary Sinus in Apolipoprotein E deficient mice
Abstract
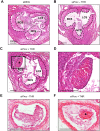
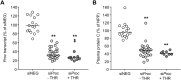
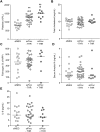
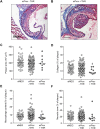
Silencing of anticoagulant protein C using RNA interference (siProc) evokes low incident but spontaneous atherothrombosis in the aortic root of apolipoprotein E-deficient (Apoe-/-) mice. The aims of the current study were (1) to analyze if plaque characteristics or circulating factors could be linked to atherothrombosis susceptibility, (2) to increase the incidence of atherothrombosis by transiently increasing blood pressure, and (3) to direct atherothrombosis to an additional predefined vascular site by applying a semi-constrictive collar around the carotid artery. siProc-driven spontaneous atherothrombosis in the aortic root of Apoe-/- mice was reproduced and occurred at an incidence of 23% (9 out of 39 mice), while the incidence of collar-induced atherothrombosis in the carotid artery was 2.6% (1 out of 39 mice). Treatment with phenylephrine, to transiently increase blood pressure, did not increase atherothrombosis in the aortic root of the Apoe-/- mice nor in the carotid arteries with collars. Plaques in the aortic root with an associated thrombus were lower in collagen and macrophage content, and mice with atherothrombosis had significantly more circulating platelets. Plasma protein C, white blood cell counts, total cholesterol, fibrinogen, serum amyloid A, and IL-6 were not different amongst siProc treated mice with or without thrombosis. Remarkably, our data revealed that thrombus formation preferably occurred on plaques in the right coronary sinus of the aortic root. In conclusion, there is a predilection of low protein C-induced spontaneous atherothrombosis in Apoe-/- mice for the right coronary sinus, a process that is associated with an increase in platelets and plaques lower in collagen and macrophage content.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Atherothrombosis model by silencing of protein C in APOE*3-Leiden.CETP transgenic mice.J Thromb Thrombolysis. 2021 Oct;52(3):715-719. doi: 10.1007/s11239-021-02488-2. Epub 2021 May 30. J Thromb Thrombolysis. 2021. PMID: 34052976 Free PMC article.
-
Silencing of Anticoagulant Protein C Evokes Low-Incident but Spontaneous Atherothrombosis in Apolipoprotein E-Deficient Mice-Brief Report.Arterioscler Thromb Vasc Biol. 2017 May;37(5):782-785. doi: 10.1161/ATVBAHA.117.309188. Epub 2017 Mar 16. Arterioscler Thromb Vasc Biol. 2017. PMID: 28302625
-
Factor XI regulates pathological thrombus formation on acutely ruptured atherosclerotic plaques.Arterioscler Thromb Vasc Biol. 2014 Aug;34(8):1668-73. doi: 10.1161/ATVBAHA.114.303209. Epub 2014 Jun 19. Arterioscler Thromb Vasc Biol. 2014. PMID: 24947525
-
[Pathology of AtheroThrombosIS (ATIS)].Drugs. 2010 Nov 1;70 Suppl 1:3-8. doi: 10.2165/00000004-000000000-00000. Drugs. 2010. PMID: 20977286 Review. Japanese.
-
Tissue Factor and Atherothrombosis.J Atheroscler Thromb. 2015;22(6):543-9. doi: 10.5551/jat.30940. Epub 2015 May 27. J Atheroscler Thromb. 2015. PMID: 26016513 Review.
Cited by
-
Atherothrombosis model by silencing of protein C in APOE*3-Leiden.CETP transgenic mice.J Thromb Thrombolysis. 2021 Oct;52(3):715-719. doi: 10.1007/s11239-021-02488-2. Epub 2021 May 30. J Thromb Thrombolysis. 2021. PMID: 34052976 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

