Insensitivity to Pain upon Adult-Onset Deletion of Nav1.7 or Its Blockade with Selective Inhibitors
- PMID: 30301756
- PMCID: PMC6596201
- DOI: 10.1523/JNEUROSCI.1049-18.2018
Insensitivity to Pain upon Adult-Onset Deletion of Nav1.7 or Its Blockade with Selective Inhibitors
Abstract
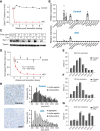
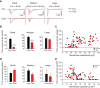
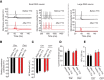
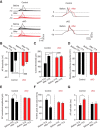
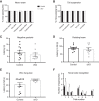
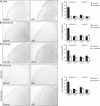
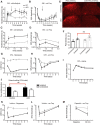
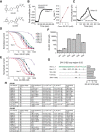
Strong human genetic evidence points to an essential contribution of the voltage-gated sodium channel Nav1.7 to pain sensation: loss of Nav1.7 function leads to congenital insensitivity to pain, whereas gain-of-function mutations in the SCN9A gene that encodes Nav1.7 cause painful neuropathies, such as inherited erythromelalgia, a syndrome characterized by episodic spontaneous pain. Selective Nav1.7 channel blockers thus hold promise as potential painkillers with improved safety and reduced unwanted side effects compared with existing therapeutics. To determine the maximum effect of a theoretically perfectly selective Nav1.7 inhibitor, we generated a tamoxifen-inducible KO mouse model enabling genetic deletion of Nav1.7 from adult mice. Electrophysiological recordings of sensory neurons from these mice following tamoxifen injection demonstrated the loss of Nav1.7 channel current and the resulting decrease in neuronal excitability of small-diameter neurons. We found that behavioral responses to most, but surprisingly not all, modalities of noxious stimulus are abolished following adult deletion of Nav1.7, pointing toward indications where Nav1.7 blockade should be efficacious. Furthermore, we demonstrate that isoform-selective acylsulfonamide Nav1.7 inhibitors show robust analgesic and antinociceptive activity acutely after a single dose in mouse pain models shown to be Nav1.7-dependent. All experiments were done with both male and female mice. Collectively, these data expand the depth of knowledge surrounding Nav1.7 biology as it relates to pain, and provide preclinical proof of efficacy that lays a clear path toward translation for the therapeutic use of Nav1.7-selective inhibitors in humans.SIGNIFICANCE STATEMENT Loss-of-function mutations in the sodium channel Nav1.7 cause congenital insensitivity to pain in humans, making Nav1.7 a top target for novel pain drugs. Targeting Nav1.7 selectively has been challenging, however, in part due to uncertainties in which rodent pain models are dependent on Nav1.7. We have developed and characterized an adult-onset Nav1.7 KO mouse model that allows us to determine the expected effects of a theoretically perfect Nav1.7 blocker. Importantly, many commonly used pain models, such as mechanical allodynia after nerve injury, appear to not be dependent on Nav1.7 in the adult. By defining which models are Nav1.7 dependent, we demonstrate that selective Nav1.7 inhibitors can approximate the effects of genetic loss of function, which previously has not been directly established.
Keywords: Nav1.7; acylsulfonamide; congenital insensitivity to pain; drug development; pain; tamoxifen.
Copyright © 2018 the authors 0270-6474/18/3810180-22$15.00/0.
Figures



Similar articles
-
[Pain and analgesia : Mutations of voltage-gated sodium channels].Schmerz. 2017 Feb;31(1):14-22. doi: 10.1007/s00482-016-0139-0. Schmerz. 2017. PMID: 27402262 German.
-
Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel Nav1.7.Nat Commun. 2015 Dec 4;6:8967. doi: 10.1038/ncomms9967. Nat Commun. 2015. PMID: 26634308 Free PMC article.
-
Nav1.7-A1632G Mutation from a Family with Inherited Erythromelalgia: Enhanced Firing of Dorsal Root Ganglia Neurons Evoked by Thermal Stimuli.J Neurosci. 2016 Jul 13;36(28):7511-22. doi: 10.1523/JNEUROSCI.0462-16.2016. J Neurosci. 2016. PMID: 27413160 Free PMC article.
-
Human Mendelian pain disorders: a key to discovery and validation of novel analgesics.Clin Genet. 2012 Oct;82(4):367-73. doi: 10.1111/j.1399-0004.2012.01942.x. Epub 2012 Aug 13. Clin Genet. 2012. PMID: 22845492 Review.
-
Pain behavior in SCN9A (Nav1.7) and SCN10A (Nav1.8) mutant rodent models.Neurosci Lett. 2021 May 14;753:135844. doi: 10.1016/j.neulet.2021.135844. Epub 2021 Mar 26. Neurosci Lett. 2021. PMID: 33775738 Review.
Cited by
-
A Novel Antigen Design Strategy to Isolate Single-Domain Antibodies that Target Human Nav1.7 and Reduce Pain in Animal Models.Adv Sci (Weinh). 2024 Oct;11(40):e2405432. doi: 10.1002/advs.202405432. Epub 2024 Aug 29. Adv Sci (Weinh). 2024. PMID: 39206821 Free PMC article.
-
Computational design of peptides to target NaV1.7 channel with high potency and selectivity for the treatment of pain.Elife. 2022 Dec 28;11:e81727. doi: 10.7554/eLife.81727. Elife. 2022. PMID: 36576241 Free PMC article.
-
Cell specific regulation of NaV1.7 activity and trafficking in rat nodose ganglia neurons.Neurobiol Pain. 2022 Nov 12;12:100109. doi: 10.1016/j.ynpai.2022.100109. eCollection 2022 Aug-Dec. Neurobiol Pain. 2022. PMID: 36531612 Free PMC article.
-
Fibroblast growth factor homologous factor 2 attenuates excitability of DRG neurons.J Neurophysiol. 2022 Nov 1;128(5):1258-1266. doi: 10.1152/jn.00361.2022. Epub 2022 Oct 12. J Neurophysiol. 2022. PMID: 36222860 Free PMC article.
-
Ion channels and pain in Fabry disease.Mol Pain. 2021 Jan-Dec;17:17448069211033172. doi: 10.1177/17448069211033172. Mol Pain. 2021. PMID: 34284652 Free PMC article. Review.
References
-
- Ahmad S, Dahllund L, Eriksson AB, Hellgren D, Karlsson U, Lund PE, Meijer IA, Meury L, Mills T, Moody A, Morinville A, Morten J, O'Donnell D, Raynoschek C, Salter H, Rouleau GA, Krupp JJ (2007) A stop codon mutation in SCN9A causes lack of pain sensation. Hum Mol Genet 16:2114–2121. 10.1093/hmg/ddm160 - DOI - PubMed
-
- Ahuja S, Mukund S, Deng L, Khakh K, Chang E, Ho H, Shriver S, Young C, Lin S, Johnson JP Jr, Wu P, Li J, Coons M, Tam C, Brillantes B, Sampang H, Mortara K, Bowman KK, Clark KR, Estevez A, et al. (2015) Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science 350:aac5464. 10.1126/science.aac5464 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
