Accelerated vs. conventionally fractionated adjuvant radiotherapy in high-risk head and neck cancer: a meta-analysis
- PMID: 30286777
- PMCID: PMC6172789
- DOI: 10.1186/s13014-018-1133-8
Accelerated vs. conventionally fractionated adjuvant radiotherapy in high-risk head and neck cancer: a meta-analysis
Abstract
Background: Adjuvant radiotherapy in advanced head and neck squamous cell cancer (HNSCC) reduces the risk of local-regional failure and most likely increases the survival rate. Patients at high risk for tumor recurrence may benefit from more aggressive altered fractionation schedules in order to reduce the overall time from surgery to completion of radiotherapy. Here, we reviewed the results of six randomized trials addressing the above hypothesis.
Methods: In the six trials of interest, a total of 988 patients with locally advanced HNSCC were randomly assigned to receive either accelerated or conventionally fractionated adjuvant radiotherapy. Hazard ratios (HR) were extracted from available publications for local-regional control, distant metastasis as well as overall-, cancer specific- and disease-free survival. Meta-analysis of the effect sizes was performed using fixed and random effect models. Acute and late side effects were categorized and summarized for comparison.
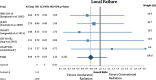
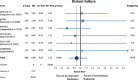
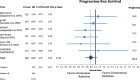
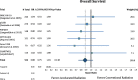
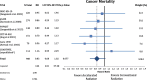
Results: Accelerated radiotherapy did not improve the loco-regional control (n = 988, HR = 0.740, CI = 0.48-1.13, p = 0.162), progression-free survival (HR = 0.89, CI = 0.76-1.04, p = 0.132) or overall survival (HR = 0.88, CI = 0.75-1.04, p = 0.148) significantly. Acute confluent mucositis occurred with significant higher frequency with accelerated radiotherapy. Late side effects did not differ significantly in either group.
Conclusion: Accelerated radiotherapy does not result in a significant improvement of loco-regional control or overall survival in high-risk patients. Acute but not late radiation toxicity were more frequent with the accelerated RT technique. In clinical practice accelerated postoperative radiation therapy might be a suitable option only for a subset of patients.
Keywords: Accelerated fractionation; Adjuvant therapy; Conventional fractionation; Head and neck cancer; High risk; Radiation therapy.
Conflict of interest statement
Ethics approval and consent to participate
There was no ethics approval necessary because in this meta-analysis we were pulling numbers from the published manuscripts and pooling results.
Consent for publication
Not applicable because in this meta-analysis we were pulling numbers from the published manuscripts and pooling results.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
A randomised trial of accelerated and conventional radiotherapy for stage III and IV squamous carcinoma of the head and neck: a Trans-Tasman Radiation Oncology Group Study.Radiother Oncol. 2001 Aug;60(2):113-22. doi: 10.1016/s0167-8140(01)00347-4. Radiother Oncol. 2001. PMID: 11439206 Clinical Trial.
-
Five versus six fractions of radiotherapy per week for squamous-cell carcinoma of the head and neck (IAEA-ACC study): a randomised, multicentre trial.Lancet Oncol. 2010 Jun;11(6):553-60. doi: 10.1016/S1470-2045(10)70072-3. Epub 2010 Apr 8. Lancet Oncol. 2010. PMID: 20382075 Clinical Trial.
-
Evidence-based radiation oncology in head and neck squamous cell carcinoma.Radiother Oncol. 2007 Oct;85(1):156-70. doi: 10.1016/j.radonc.2007.04.002. Epub 2007 May 4. Radiother Oncol. 2007. PMID: 17482300 Review.
-
Randomized phase I/II trial of two variants of accelerated fractionated radiotherapy regimens for advanced head and neck cancer: results of RTOG 88-09.Int J Radiat Oncol Biol Phys. 1995 Jun 15;32(3):589-97. doi: 10.1016/0360-3016(95)00078-D. Int J Radiat Oncol Biol Phys. 1995. PMID: 7790243 Clinical Trial.
-
Systematic Review and Meta-analysis of Conventionally Fractionated Concurrent Chemoradiotherapy versus Altered Fractionation Radiotherapy Alone in the Definitive Management of Locoregionally Advanced Head and Neck Squamous Cell Carcinoma.Clin Oncol (R Coll Radiol). 2016 Jan;28(1):50-61. doi: 10.1016/j.clon.2015.09.002. Epub 2015 Oct 9. Clin Oncol (R Coll Radiol). 2016. PMID: 26454839 Review.
Cited by
-
Randomised clinical trial on 7-days-a-week postoperative radiotherapy vs. concurrent postoperative radio-chemotherapy in locally advanced cancer of the oral cavity/oropharynx.Br J Radiol. 2020 Dec 1;93(1116):20200288. doi: 10.1259/bjr.20200288. Epub 2020 Sep 30. Br J Radiol. 2020. PMID: 32960662 Free PMC article. Clinical Trial.
-
Definitive chemoradiotherapy in patients with squamous cell cancers of the head and neck - results from an unselected cohort of the clinical cooperation group "Personalized Radiotherapy in Head and Neck Cancer".Radiat Oncol. 2020 Jan 6;15(1):7. doi: 10.1186/s13014-019-1452-4. Radiat Oncol. 2020. PMID: 31906998 Free PMC article.
-
Systematic review of postoperative therapy for resected squamous cell carcinoma of the head and neck: Executive summary of the American Radium Society appropriate use criteria.Head Neck. 2021 Jan;43(1):367-391. doi: 10.1002/hed.26490. Epub 2020 Oct 23. Head Neck. 2021. PMID: 33098180 Free PMC article.
-
Accelerated Hyperfractionated Radiotherapy versus Conventional Fractionation Radiotherapy for Head and Neck Cancer: A Meta-Analysis of Randomized Controlled Trials.J Oncol. 2019 Nov 28;2019:7634746. doi: 10.1155/2019/7634746. eCollection 2019. J Oncol. 2019. PMID: 31885584 Free PMC article.
-
Addition of chemotherapy to hyperfractionated radiotherapy in advanced head and neck cancer-a meta-analysis.Strahlenther Onkol. 2019 Dec;195(12):1041-1049. doi: 10.1007/s00066-019-01511-z. Epub 2019 Oct 4. Strahlenther Onkol. 2019. PMID: 31586229 Review. English.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

