Basement membranes in the cornea and other organs that commonly develop fibrosis
- PMID: 30284084
- PMCID: PMC6258348
- DOI: 10.1007/s00441-018-2934-7
Basement membranes in the cornea and other organs that commonly develop fibrosis
Abstract
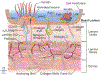
Basement membranes are thin connective tissue structures composed of organ-specific assemblages of collagens, laminins, proteoglycan-like perlecan, nidogens, and other components. Traditionally, basement membranes are thought of as structures which primarily function to anchor epithelial, endothelial, or parenchymal cells to underlying connective tissues. While this role is important, other functions such as the modulation of growth factors and cytokines that regulate cell proliferation, migration, differentiation, and fibrosis are equally important. An example of this is the critical role of both the epithelial basement membrane and Descemet's basement membrane in the cornea in modulating myofibroblast development and fibrosis, as well as myofibroblast apoptosis and the resolution of fibrosis. This article compares the ultrastructure and functions of key basement membranes in several organs to illustrate the variability and importance of these structures in organs that commonly develop fibrosis.
Keywords: Basement membrane; Cornea; Fibrosis; Lung; Skin.
Figures





Similar articles
-
The Corneal Basement Membranes and Stromal Fibrosis.Invest Ophthalmol Vis Sci. 2018 Aug 1;59(10):4044-4053. doi: 10.1167/iovs.18-24428. Invest Ophthalmol Vis Sci. 2018. PMID: 30098200 Free PMC article. Review.
-
Defective perlecan-associated basement membrane regeneration and altered modulation of transforming growth factor beta in corneal fibrosis.Cell Mol Life Sci. 2022 Feb 21;79(3):144. doi: 10.1007/s00018-022-04184-7. Cell Mol Life Sci. 2022. PMID: 35188596 Free PMC article. Review.
-
Fibrosis Is a Basement Membrane-Related Disease in the Cornea: Injury and Defective Regeneration of Basement Membranes May Underlie Fibrosis in Other Organs.Cells. 2022 Jan 17;11(2):309. doi: 10.3390/cells11020309. Cells. 2022. PMID: 35053425 Free PMC article. Review.
-
Corneal epithelial basement membrane: Structure, function and regeneration.Exp Eye Res. 2020 May;194:108002. doi: 10.1016/j.exer.2020.108002. Epub 2020 Mar 13. Exp Eye Res. 2020. PMID: 32179076 Free PMC article. Review.
-
Injury and defective regeneration of the epithelial basement membrane in corneal fibrosis: A paradigm for fibrosis in other organs?Matrix Biol. 2017 Dec;64:17-26. doi: 10.1016/j.matbio.2017.06.003. Epub 2017 Jun 15. Matrix Biol. 2017. PMID: 28625845 Free PMC article. Review.
Cited by
-
Safety and efficacy of combination of suberoylamilide hydroxyamic acid and mitomycin C in reducing pro-fibrotic changes in human corneal epithelial cells.Sci Rep. 2021 Feb 23;11(1):4392. doi: 10.1038/s41598-021-83881-y. Sci Rep. 2021. PMID: 33623133 Free PMC article.
-
USP10 Targeted Self-Deliverable siRNA to Prevent Scarring in the Cornea.Mol Ther Nucleic Acids. 2020 Sep 4;21:1029-1043. doi: 10.1016/j.omtn.2020.07.032. Epub 2020 Jul 25. Mol Ther Nucleic Acids. 2020. PMID: 32829179 Free PMC article.
-
Increased Fibrosis in a Mouse Model of Anti-Laminin 332 Mucous Membrane Pemphigoid Remains Unaltered by Inhibition of Aldehyde Dehydrogenase.Front Immunol. 2022 Feb 7;12:812627. doi: 10.3389/fimmu.2021.812627. eCollection 2021. Front Immunol. 2022. PMID: 35197965 Free PMC article.
-
The Yin and Yang of Mesenchymal Cells in the Corneal Stromal Fibrosis Response to Injury: The Cornea as a Model of Fibrosis in Other Organs.Biomolecules. 2022 Dec 31;13(1):87. doi: 10.3390/biom13010087. Biomolecules. 2022. PMID: 36671472 Free PMC article. Review.
-
Extracellular matrix dynamics: tracking in biological systems and their implications.J Biol Eng. 2022 May 30;16(1):13. doi: 10.1186/s13036-022-00292-x. J Biol Eng. 2022. PMID: 35637526 Free PMC article. Review.
References
-
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y (1999) Perlecan is essential for cartilage and cephalic development. Nat Genet 23:354–358 - PubMed
-
- Aumailley M, Battaglia C, Mayer U, Reinhardt D, Nischt R, Timpl R, Fox JW (1993) Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int 43:7–12 - PubMed
-
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD (2005) A simplified laminin nomenclature. Matrix biology : journal of the International Society for Matrix Biology 24:326–332 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

