Potential application of cell reprogramming techniques for cancer research
- PMID: 30283976
- PMCID: PMC6326983
- DOI: 10.1007/s00018-018-2924-7
Potential application of cell reprogramming techniques for cancer research
Abstract
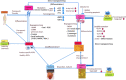
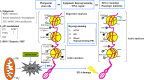
The ability to control the transition from an undifferentiated stem cell to a specific cell fate is one of the key techniques that are required for the application of interventional technologies to regenerative medicine and the treatment of tumors and metastases and of neurodegenerative diseases. Reprogramming technologies, which include somatic cell nuclear transfer, induced pluripotent stem cells, and the direct reprogramming of specific cell lineages, have the potential to alter cell plasticity in translational medicine for cancer treatment. The characterization of cancer stem cells (CSCs), the identification of oncogene and tumor suppressor genes for CSCs, and the epigenetic study of CSCs and their microenvironments are important topics. This review summarizes the application of cell reprogramming technologies to cancer modeling and treatment and discusses possible obstacles, such as genetic and epigenetic alterations in cancer cells, as well as the strategies that can be used to overcome these obstacles to cancer research.
Keywords: Cancer stem cells; Epigenetics; Induced pluripotent stem cells; Organoid culture; Reactive oxygen species; Somatic cell nuclear transfer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Reprogramming of retinoblastoma cancer cells into cancer stem cells.Biochem Biophys Res Commun. 2017 Jan 22;482(4):549-555. doi: 10.1016/j.bbrc.2016.11.072. Epub 2016 Nov 14. Biochem Biophys Res Commun. 2017. PMID: 27856246
-
Application of Cancer Cell Reprogramming Technology to Human Cancer Research.Anticancer Res. 2017 Jul;37(7):3367-3377. doi: 10.21873/anticanres.11703. Anticancer Res. 2017. PMID: 28668824 Review.
-
Linking Pluripotency Reprogramming and Cancer.Stem Cells Transl Med. 2017 Feb;6(2):335-339. doi: 10.5966/sctm.2015-0225. Epub 2016 Sep 20. Stem Cells Transl Med. 2017. PMID: 28191771 Free PMC article.
-
Reprogramming of cancer stem cells into non-tumorigenic cells using stem cell exosomes for cancer therapy.Biochem Biophys Res Commun. 2019 May 7;512(3):511-516. doi: 10.1016/j.bbrc.2019.03.072. Epub 2019 Mar 21. Biochem Biophys Res Commun. 2019. PMID: 30905410
-
Primitive Cancer Cell States: A Target for Drug Screening?Trends Pharmacol Sci. 2019 Mar;40(3):161-171. doi: 10.1016/j.tips.2019.01.003. Epub 2019 Jan 29. Trends Pharmacol Sci. 2019. PMID: 30709543 Review.
Cited by
-
Biomarkers of Cancer Stem Cells for Experimental Research and Clinical Application.J Pers Med. 2022 Apr 29;12(5):715. doi: 10.3390/jpm12050715. J Pers Med. 2022. PMID: 35629138 Free PMC article. Review.
-
Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy.Mol Cancer. 2023 Nov 28;22(1):189. doi: 10.1186/s12943-023-01873-0. Mol Cancer. 2023. PMID: 38017433 Free PMC article. Review.
-
Using Citation Context to Improve the Retrieval of Research Article from Cancer Research Journals.Asian Pac J Cancer Prev. 2019 Mar 26;20(3):951-960. doi: 10.31557/APJCP.2019.20.3.951. Asian Pac J Cancer Prev. 2019. PMID: 30912420 Free PMC article.
-
Translational models of 3-D organoids and cancer stem cells in gastric cancer research.Stem Cell Res Ther. 2021 Sep 6;12(1):492. doi: 10.1186/s13287-021-02521-4. Stem Cell Res Ther. 2021. PMID: 34488885 Free PMC article. Review.
-
Ribosome induces transdifferentiation of A549 and H-111-TC cancer cell lines.Biochem Biophys Rep. 2021 Feb 12;26:100946. doi: 10.1016/j.bbrep.2021.100946. eCollection 2021 Jul. Biochem Biophys Rep. 2021. PMID: 33644423 Free PMC article.
References
-
- Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–640. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

