New Vaccine Technologies to Combat Outbreak Situations
- PMID: 30283434
- PMCID: PMC6156540
- DOI: 10.3389/fimmu.2018.01963
New Vaccine Technologies to Combat Outbreak Situations
Abstract
Ever since the development of the first vaccine more than 200 years ago, vaccinations have greatly decreased the burden of infectious diseases worldwide, famously leading to the eradication of small pox and allowing the restriction of diseases such as polio, tetanus, diphtheria, and measles. A multitude of research efforts focuses on the improvement of established and the discovery of new vaccines such as the HPV (human papilloma virus) vaccine in 2006. However, radical changes in the density, age distribution and traveling habits of the population worldwide as well as the changing climate favor the emergence of old and new pathogens that bear the risk of becoming pandemic threats. In recent years, the rapid spread of severe infections such as HIV, SARS, Ebola, and Zika have highlighted the dire need for global preparedness for pandemics, which necessitates the extremely rapid development and comprehensive distribution of vaccines against potentially previously unknown pathogens. What is more, the emergence of antibiotic resistant bacteria calls for new approaches to prevent infections. Given these changes, established methods for the identification of new vaccine candidates are no longer sufficient to ensure global protection. Hence, new vaccine technologies able to achieve rapid development as well as large scale production are of pivotal importance. This review will discuss viral vector and nucleic acid-based vaccines (DNA and mRNA vaccines) as new approaches that might be able to tackle these challenges to global health.
Keywords: DNA vaccine; mRNA vaccine; pandemics; vaccine development; viral vector vaccine.
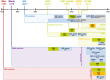
Figures
Similar articles
-
Vaccines: Shaping global health.Vaccine. 2017 Mar 14;35(12):1579-1585. doi: 10.1016/j.vaccine.2017.02.017. Epub 2017 Feb 23. Vaccine. 2017. PMID: 28237501 Free PMC article.
-
Funding vaccines for emerging infectious diseases.Hum Vaccin Immunother. 2018 Jul 3;14(7):1760-1762. doi: 10.1080/21645515.2017.1412024. Epub 2018 Jan 16. Hum Vaccin Immunother. 2018. PMID: 29194012 Free PMC article.
-
Outbreak response as an essential component of vaccine development.Lancet Infect Dis. 2019 Nov;19(11):e399-e403. doi: 10.1016/S1473-3099(19)30305-6. Epub 2019 Jun 27. Lancet Infect Dis. 2019. PMID: 31256955 Free PMC article. Review.
-
Strengthening global vaccine access for adolescents and adults.Vaccine. 2017 Dec 14;35(49 Pt B):6823-6827. doi: 10.1016/j.vaccine.2017.10.023. Epub 2017 Nov 6. Vaccine. 2017. PMID: 29122384 Review.
-
Vaccines, our shared responsibility.Vaccine. 2015 May 5;33(19):2197-2202. doi: 10.1016/j.vaccine.2015.02.065. Epub 2015 Mar 4. Vaccine. 2015. PMID: 25749248
Cited by
-
Prevention of COVID-19: Preventive Strategies for General Population, Healthcare Setting, and Various Professions.Adv Exp Med Biol. 2021;1318:575-604. doi: 10.1007/978-3-030-63761-3_32. Adv Exp Med Biol. 2021. PMID: 33973200
-
Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: A review.Int J Biol Macromol. 2021 Jul 1;182:648-658. doi: 10.1016/j.ijbiomac.2021.04.005. Epub 2021 Apr 16. Int J Biol Macromol. 2021. PMID: 33862071 Free PMC article. Review.
-
Current Status of COVID-19 (Pre)Clinical Vaccine Development.Angew Chem Int Ed Engl. 2020 Oct 19;59(43):18885-18897. doi: 10.1002/anie.202008319. Epub 2020 Sep 2. Angew Chem Int Ed Engl. 2020. PMID: 32663348 Free PMC article. Review.
-
Lessons from SARS-CoV-2 Pandemic: Evolution, Disease Dynamics and Future.Biology (Basel). 2020 Jun 26;9(6):141. doi: 10.3390/biology9060141. Biology (Basel). 2020. PMID: 32604825 Free PMC article. Review.
-
Towards personalized vaccines.Front Immunol. 2024 Oct 3;15:1436108. doi: 10.3389/fimmu.2024.1436108. eCollection 2024. Front Immunol. 2024. PMID: 39421749 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

