Exploring the impact of inoculum dose on host immunity and morbidity to inform model-based vaccine design
- PMID: 30273336
- PMCID: PMC6181424
- DOI: 10.1371/journal.pcbi.1006505
Exploring the impact of inoculum dose on host immunity and morbidity to inform model-based vaccine design
Abstract
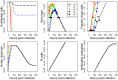
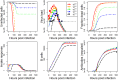
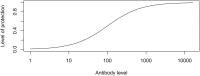
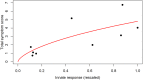
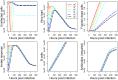
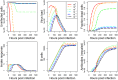
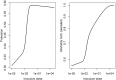
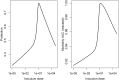
Vaccination is an effective method to protect against infectious diseases. An important consideration in any vaccine formulation is the inoculum dose, i.e., amount of antigen or live attenuated pathogen that is used. Higher levels generally lead to better stimulation of the immune response but might cause more severe side effects and allow for less population coverage in the presence of vaccine shortages. Determining the optimal amount of inoculum dose is an important component of rational vaccine design. A combination of mathematical models with experimental data can help determine the impact of the inoculum dose. We illustrate the concept of using data and models to inform inoculum dose determination for vaccines, wby fitting a mathematical model to data from influenza A virus (IAV) infection of mice and human parainfluenza virus (HPIV) infection of cotton rats at different inoculum doses. We use the model to map inoculum dose to the level of immune protection and morbidity and to explore how such a framework might be used to determine an optimal inoculum dose. We show how a framework that combines mathematical models with experimental data can be used to study the impact of inoculum dose on important outcomes such as immune protection and morbidity. Our findings illustrate that the impact of inoculum dose on immune protection and morbidity can depend on the specific pathogen and that both protection and morbidity do not necessarily increase monotonically with increasing inoculum dose. Once vaccine design goals are specified with required levels of protection and acceptable levels of morbidity, our proposed framework can help in the rational design of vaccines and determination of the optimal amount of inoculum.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
A strategic approach to vaccine development: animal models, monitoring vaccine efficacy, formulation and delivery.Adv Drug Deliv Rev. 2002 Oct 4;54(6):851-61. doi: 10.1016/s0169-409x(02)00072-8. Adv Drug Deliv Rev. 2002. PMID: 12363434 Review.
-
Modeling inoculum dose dependent patterns of acute virus infections.J Theor Biol. 2014 Apr 21;347:63-73. doi: 10.1016/j.jtbi.2014.01.008. Epub 2014 Jan 15. J Theor Biol. 2014. PMID: 24440713
-
A systems framework for vaccine design.Curr Opin Immunol. 2013 Oct;25(5):551-5. doi: 10.1016/j.coi.2013.09.014. Curr Opin Immunol. 2013. PMID: 24358511 Free PMC article. Review.
-
A Single-Dose Recombinant Parainfluenza Virus 5-Vectored Vaccine Expressing Respiratory Syncytial Virus (RSV) F or G Protein Protected Cotton Rats and African Green Monkeys from RSV Challenge.J Virol. 2017 May 12;91(11):e00066-17. doi: 10.1128/JVI.00066-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28298602 Free PMC article.
-
Principles of Vaccination.Methods Mol Biol. 2016;1403:57-84. doi: 10.1007/978-1-4939-3387-7_3. Methods Mol Biol. 2016. PMID: 27076125
Cited by
-
From Bench to Field: A Guide to Formulating and Evaluating Anti-Tick Vaccines Delving beyond Efficacy to Effectiveness.Vaccines (Basel). 2021 Oct 15;9(10):1185. doi: 10.3390/vaccines9101185. Vaccines (Basel). 2021. PMID: 34696291 Free PMC article. Review.
-
Estimation of viral kinetics model parameters in young and aged SARS-CoV-2 infected macaques.R Soc Open Sci. 2021 Nov 17;8(11):202345. doi: 10.1098/rsos.202345. eCollection 2021 Nov. R Soc Open Sci. 2021. PMID: 34804559 Free PMC article.
-
Response Type and Host Species may be Sufficient to Predict Dose-Response Curve Shape for Adenoviral Vector Vaccines.Vaccines (Basel). 2020 Mar 30;8(2):155. doi: 10.3390/vaccines8020155. Vaccines (Basel). 2020. PMID: 32235634 Free PMC article.
-
Uncovering critical properties of the human respiratory syncytial virus by combining in vitro assays and in silico analyses.PLoS One. 2019 Apr 15;14(4):e0214708. doi: 10.1371/journal.pone.0214708. eCollection 2019. PLoS One. 2019. PMID: 30986239 Free PMC article.
-
Symptom propagation in respiratory pathogens of public health concern: a review of the evidence.J R Soc Interface. 2024 Jul;21(216):20240009. doi: 10.1098/rsif.2024.0009. Epub 2024 Jul 24. J R Soc Interface. 2024. PMID: 39045688 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

