Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood-brain barrier model
- PMID: 30264400
- PMCID: PMC6282717
- DOI: 10.1111/epi.14567
Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood-brain barrier model
Abstract
Objective: Nuclear receptors and cytochrome P450 (CYP) regulate hepatic metabolism of several drugs. Nuclear receptors are expressed at the neurovascular unit of patients with drug-resistant epilepsy. We studied whether glucocorticoid receptor (GR) silencing or inhibition in human epileptic brain endothelial cells (EPI-ECs) functionally impacts drug bioavailability across an in vitro model of the blood-brain barrier (BBB) by CYP-multidrug transporter (multidrug resistance protein 1, MDR1) mechanisms.
Methods: Surgically resected brain specimens from patients with drug-resistant epilepsy, primary EPI-ECs, and control human brain microvascular endothelial cells (HBMECs) were used. Expression of GR, pregnane X receptor, CYP3A4, and MDR1 was analyzed pre- and post-GR silencing in EPI-ECs. Endothelial cells were co-cultured with astrocytes and seeded in an in vitro flow-based BBB model (DIV-BBB). Alternatively, the GR inhibitor mifepristone was added to the EPI-EC DIV-BBB. Integrity of the BBB was monitored by measuring transendothelial electrical resistance. Cell viability was assessed by glucose-lactate levels. Permeability of [3 H]sucrose and [14 C]phenytoin was quantified. CYP function was determined by measuring resorufin formation and oxcarbazepine (OXC) metabolism.
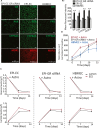
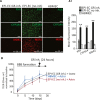
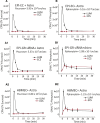
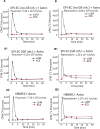
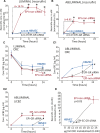
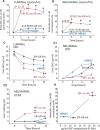
Results: Silencing and inhibition of GR in EPI-ECs resulted in decreased pregnane X receptor, CYP3A4, and MDR1 expression. GR silencing or inhibition did not affect BBB properties in vitro, as transendothelial electrical resistance and Psucrose were unaltered, and glucose metabolism was maintained. GR EPI-EC silencing or inhibition led to (1) increased Pphenytoin BBB permeability as compared to control; (2) decreased CYP function, indirectly evaluated by resorufin formation; (3) improved OXC bioavailability with increased abluminal (brain-side) OXC levels as compared to control.
Significance: Our results suggest that modulating GR expression in EPI-ECs at the BBB modifies drug metabolism and penetration by a mechanism encompassing P450 and efflux transporters. The latter could be exploited for future drug design and to overcome pharmacoresistance.
Keywords: P450 enzymes; bioavailability; drug-resistant epilepsy; multidrug transporter; nuclear receptors.
© 2018 The Authors. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Figures
Similar articles
-
Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells.Epilepsia. 2017 Apr;58(4):576-585. doi: 10.1111/epi.13703. Epub 2017 Feb 15. Epilepsia. 2017. PMID: 28199000 Free PMC article.
-
Heat Shock Proteins Accelerate the Maturation of Brain Endothelial Cell Glucocorticoid Receptor in Focal Human Drug-Resistant Epilepsy.Mol Neurobiol. 2020 Nov;57(11):4511-4529. doi: 10.1007/s12035-020-02043-9. Epub 2020 Aug 3. Mol Neurobiol. 2020. PMID: 32748370 Free PMC article.
-
Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs.Epilepsia. 2007 Mar;48(3):505-16. doi: 10.1111/j.1528-1167.2006.00960.x. Epub 2007 Feb 22. Epilepsia. 2007. PMID: 17326793
-
Do ATP-binding cassette transporters cause pharmacoresistance in epilepsy? Problems and approaches in determining which antiepileptic drugs are affected.Curr Pharm Des. 2011;17(26):2808-28. doi: 10.2174/138161211797440212. Curr Pharm Des. 2011. PMID: 21827408 Review.
-
Vascular and parenchymal mechanisms in multiple drug resistance: a lesson from human epilepsy.Curr Drug Targets. 2003 May;4(4):297-304. doi: 10.2174/1389450033491109. Curr Drug Targets. 2003. PMID: 12699350 Review.
Cited by
-
Neurovascular Drug Biotransformation Machinery in Focal Human Epilepsies: Brain CYP3A4 Correlates with Seizure Frequency and Antiepileptic Drug Therapy.Mol Neurobiol. 2019 Dec;56(12):8392-8407. doi: 10.1007/s12035-019-01673-y. Epub 2019 Jun 26. Mol Neurobiol. 2019. PMID: 31243719 Free PMC article.
-
In vitro Models of the Blood-Brain Barrier: Tools in Translational Medicine.Front Med Technol. 2021 Feb 15;2:623950. doi: 10.3389/fmedt.2020.623950. eCollection 2020. Front Med Technol. 2021. PMID: 35047899 Free PMC article. Review.
-
Glucocorticoid Receptor β Isoform Predominates in the Human Dysplastic Brain Region and Is Modulated by Age, Sex, and Antiseizure Medication.Int J Mol Sci. 2022 Apr 29;23(9):4940. doi: 10.3390/ijms23094940. Int J Mol Sci. 2022. PMID: 35563330 Free PMC article.
-
Drug Delivery Challenges in Brain Disorders across the Blood-Brain Barrier: Novel Methods and Future Considerations for Improved Therapy.Biomedicines. 2021 Dec 4;9(12):1834. doi: 10.3390/biomedicines9121834. Biomedicines. 2021. PMID: 34944650 Free PMC article. Review.
-
Neurovascular glucocorticoid receptors and glucocorticoids: implications in health, neurological disorders and drug therapy.Drug Discov Today. 2020 Jan;25(1):89-106. doi: 10.1016/j.drudis.2019.09.009. Epub 2019 Sep 18. Drug Discov Today. 2020. PMID: 31541713 Free PMC article. Review.
References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte‐endothelial interactions at the blood‐brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Oby E, Janigro D. The blood‐brain barrier and epilepsy. Epilepsia. 2006;47:1761–74. - PubMed
-
- Pardridge WM. Molecular biology of the blood‐brain barrier. Mol Biotechnol. 2005;30:57–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

