Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance
- PMID: 30262932
- PMCID: PMC6152896
- DOI: 10.1016/j.ccr.2018.04.007
Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance
Abstract
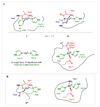
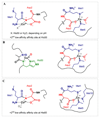
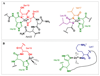
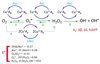
Several diseases share misfolding of different peptides and proteins as a key feature for their development. This is the case of important neurodegenerative diseases such as Alzheimer's and Parkinson's diseases and type II diabetes mellitus. Even more, metal ions such as copper and zinc might play an important role upon interaction with amyloidogenic peptides and proteins, which could impact their aggregation and toxicity abilities. In this review, the different coordination modes proposed for copper and zinc with amyloid-β, α-synuclein and IAPP will be reviewed as well as their impact on the aggregation, and ROS production in the case of copper. In addition, a special focus will be given to the mutations that affect metal binding and lead to familial cases of the diseases. Different modifications of the peptides that have been observed in vivo and could be relevant for the coordination of metal ions are also described.
Keywords: Amyloid-β; amylin; copper; familial mutations; zinc; α-synuclein.
Conflict of interest statement
Conflicts of interest: none.
Figures

Similar articles
-
Metal ions and intrinsically disordered proteins and peptides: from Cu/Zn amyloid-β to general principles.Acc Chem Res. 2014 Aug 19;47(8):2252-9. doi: 10.1021/ar400293h. Epub 2014 May 29. Acc Chem Res. 2014. PMID: 24871565
-
Copper(I/II), α/β-Synuclein and Amyloid-β: Menage à Trois?Chembiochem. 2015 Nov 2;16(16):2319-28. doi: 10.1002/cbic.201500425. Epub 2015 Sep 25. Chembiochem. 2015. PMID: 26338312
-
Cu and Zn interactions with Aβ peptides: consequence of coordination on aggregation and formation of neurotoxic soluble Aβ oligomers.Metallomics. 2019 Jan 23;11(1):64-84. doi: 10.1039/c8mt00203g. Metallomics. 2019. PMID: 30234208 Review.
-
Zinc(II) modulates specifically amyloid formation and structure in model peptides.J Biol Inorg Chem. 2011 Feb;16(2):333-40. doi: 10.1007/s00775-010-0729-8. J Biol Inorg Chem. 2011. PMID: 21061029
-
Interaction of amylin species with transition metals and membranes.J Inorg Biochem. 2019 Feb;191:69-76. doi: 10.1016/j.jinorgbio.2018.11.004. Epub 2018 Nov 10. J Inorg Biochem. 2019. PMID: 30468944 Review.
Cited by
-
A cocktail of Cu2+- and Zn2+-peptoid-based chelators can stop ROS formation for Alzheimer's disease therapy.Chem Sci. 2024 Oct 24;15(45):18855-64. doi: 10.1039/d4sc04313h. Online ahead of print. Chem Sci. 2024. PMID: 39464602 Free PMC article.
-
Antioxidant Berberine-Derivative Inhibits Multifaceted Amyloid Toxicity.iScience. 2020 Apr 24;23(4):101005. doi: 10.1016/j.isci.2020.101005. Epub 2020 Mar 25. iScience. 2020. PMID: 32272441 Free PMC article.
-
Unveiling the impact of oxidation-driven endogenous protein interactions on the dynamics of amyloid-β aggregation and toxicity.Chem Sci. 2023 Apr 25;14(20):5340-5349. doi: 10.1039/d3sc00881a. eCollection 2023 May 24. Chem Sci. 2023. PMID: 37234895 Free PMC article.
-
The Palladium(II) Complex of Aβ4-16 as Suitable Model for Structural Studies of Biorelevant Copper(II) Complexes of N-Truncated Beta-Amyloids.Int J Mol Sci. 2020 Dec 2;21(23):9200. doi: 10.3390/ijms21239200. Int J Mol Sci. 2020. PMID: 33276669 Free PMC article.
-
Novel Perspective on Alzheimer's Disease Treatment: Rosmarinic Acid Molecular Interplay with Copper(II) and Amyloid β.Life (Basel). 2020 Jul 20;10(7):118. doi: 10.3390/life10070118. Life (Basel). 2020. PMID: 32698429 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
