AMPK Mediates Muscle Mass Change But Not the Transition of Myosin Heavy Chain Isoforms during Unloading and Reloading of Skeletal Muscles in Mice
- PMID: 30262782
- PMCID: PMC6212939
- DOI: 10.3390/ijms19102954
AMPK Mediates Muscle Mass Change But Not the Transition of Myosin Heavy Chain Isoforms during Unloading and Reloading of Skeletal Muscles in Mice
Abstract
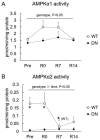
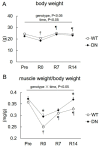
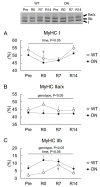
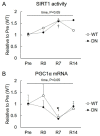
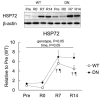
5'AMP-activated protein kinase (AMPK) plays an important role in the regulation of skeletal muscle mass and fiber-type distribution. However, it is unclear whether AMPK is involved in muscle mass change or transition of myosin heavy chain (MyHC) isoforms in response to unloading or increased loading. Here, we checked whether AMPK controls muscle mass change and transition of MyHC isoforms during unloading and reloading using mice expressing a skeletal-muscle-specific dominant-negative AMPKα1 (AMPK-DN). Fourteen days of hindlimb unloading reduced the soleus muscle weight in wild-type and AMPK-DN mice, but reduction in the muscle mass was partly attenuated in AMPK-DN mice. There was no difference in the regrown muscle weight between the mice after 7 days of reloading, and there was concomitantly reduced AMPKα2 activity, however it was higher in AMPK-DN mice after 14 days reloading. No difference was observed between the mice in relation to the levels of slow-type MyHC I, fast-type MyHC IIa/x, and MyHC IIb isoforms following unloading and reloading. The levels of 72-kDa heat-shock protein, which preserves muscle mass, increased in AMPK-DN-mice. Our results indicate that AMPK mediates the progress of atrophy during unloading and regrowth of atrophied muscles following reloading, but it does not influence the transition of MyHC isoforms.
Keywords: atrophy; fiber-type; heat shock protein; peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α); regrowth; sirtuin 1 (SIRT1).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Rapid decline in MyHC I(β) mRNA expression in rat soleus during hindlimb unloading is associated with AMPK dephosphorylation.J Physiol. 2017 Dec 1;595(23):7123-7134. doi: 10.1113/JP275184. Epub 2017 Oct 25. J Physiol. 2017. PMID: 28975644 Free PMC article.
-
The involvement of transient receptor potential canonical type 1 in skeletal muscle regrowth after unloading-induced atrophy.J Physiol. 2016 Jun 1;594(11):3111-26. doi: 10.1113/JP271705. Epub 2016 Feb 4. J Physiol. 2016. PMID: 26752511 Free PMC article.
-
Cellular adaptations in soleus muscle during recovery after hindlimb unloading.Acta Physiol (Oxf). 2008 Mar;192(3):381-95. doi: 10.1111/j.1748-1716.2007.01747.x. Epub 2007 Sep 24. Acta Physiol (Oxf). 2008. PMID: 17892520
-
AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling.Int J Mol Sci. 2018 Nov 12;19(11):3558. doi: 10.3390/ijms19113558. Int J Mol Sci. 2018. PMID: 30424476 Free PMC article. Review.
-
The Role of AMPK in Neuromuscular Biology and Disease.Trends Endocrinol Metab. 2018 May;29(5):300-312. doi: 10.1016/j.tem.2018.02.010. Epub 2018 Mar 20. Trends Endocrinol Metab. 2018. PMID: 29572064 Review.
Cited by
-
DNA-PKcs regulates myogenesis in an Akt-dependent manner independent of induced DNA damage.Cell Death Differ. 2023 Aug;30(8):1900-1915. doi: 10.1038/s41418-023-01177-2. Epub 2023 Jul 3. Cell Death Differ. 2023. PMID: 37400716 Free PMC article.
-
Metformin Pre-Treatment as a Means of Mitigating Disuse-Induced Rat Soleus Muscle Wasting.Curr Issues Mol Biol. 2023 Apr 4;45(4):3068-3086. doi: 10.3390/cimb45040201. Curr Issues Mol Biol. 2023. PMID: 37185725 Free PMC article.
-
Skeletal Muscle Recovery from Disuse Atrophy: Protein Turnover Signaling and Strategies for Accelerating Muscle Regrowth.Int J Mol Sci. 2020 Oct 26;21(21):7940. doi: 10.3390/ijms21217940. Int J Mol Sci. 2020. PMID: 33114683 Free PMC article. Review.
-
Neutralizing mitochondrial ROS does not rescue muscle atrophy induced by hindlimb unloading in female mice.J Appl Physiol (1985). 2020 Jul 1;129(1):124-132. doi: 10.1152/japplphysiol.00456.2019. Epub 2020 Jun 18. J Appl Physiol (1985). 2020. PMID: 32552434 Free PMC article.
-
AMP-Activated Protein Kinase Signalling.Int J Mol Sci. 2019 Feb 12;20(3):766. doi: 10.3390/ijms20030766. Int J Mol Sci. 2019. PMID: 30759716 Free PMC article.
References
-
- Yokoyama S., Ohno Y., Egawa T., Yasuhara K., Nakai A., Sugiura T., Ohira Y., Yoshioka T., Okita M., Origuchi T., et al. Heat shock transcription factor 1-associated expression of slow myosin heavy chain in mouse soleus muscle in response to unloading with or without reloading. Acta Physiol. 2016;217:325–337. doi: 10.1111/apha.12692. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

