Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair
- PMID: 30260581
- PMCID: PMC6265633
- DOI: 10.1002/sctm.18-0042
Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair
Abstract
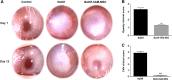
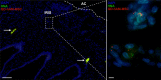
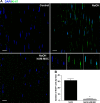
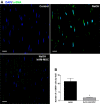
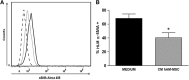
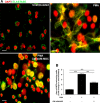
Acute ocular chemical burns are ophthalmic emergencies requiring immediate diagnosis and treatment as they may lead to permanent impairment of vision. The clinical manifestations of such burns are produced by exacerbated innate immune response via the infiltration of inflammatory cells and activation of stromal fibroblasts. New therapies are emerging that are dedicated to repair mechanisms that improve the ocular surface after damage; for example, transplantation of stem cells (SC) has been successfully reported for this purpose. The pursuit of easily accessible, noninvasive procedures to obtain SC has led researchers to focus on human tissues such as amniotic membrane. Human amniotic mesenchymal SC (hAM-MSC) inhibits proinflammatory and fibrotic processes in different diseases. hAM-MSC expresses low levels of classical MHC-I and they do not express MHC-II, making them suitable for regenerative medicine. The aim of this study was to evaluate the effect of intracameral injection of hAM-MSC on the clinical manifestations, the infiltration of inflammatory cells, and the activation of stromal fibroblasts in a corneal alkali-burn model. We also determined the in vitro effect of hAM-MSC conditioned medium (CM) on α-SMA+ human limbal myofibroblast (HLM) frequency and on release of neutrophil extracellular traps (NETs). Our results show that intracameral hAM-MSC injection reduces neovascularization, opacity, stromal inflammatory cell infiltrate, and stromal α-SMA+ cells in our model. Moreover, in in vitro assays, CM from hAM-MSC decreased the quantity of α-SMA+ HLM and the release of NETs. These results suggest that intracameral hAM-MSC injection induces an anti-inflammatory and anti-fibrotic environment that promotes corneal wound healing. Stem Cells Translational Medicine 2018;7:906-917.
Keywords: Corneal repair; Inflammation; NETs; hAM-MSC; α-SMA myofibroblasts.
© 2018 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures
Similar articles
-
Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing.Eye (Lond). 2006 Apr;20(4):482-90. doi: 10.1038/sj.eye.6701913. Eye (Lond). 2006. PMID: 15895027
-
Priming human adipose-derived mesenchymal stem cells for corneal surface regeneration.J Cell Mol Med. 2021 Jun;25(11):5124-5137. doi: 10.1111/jcmm.16501. Epub 2021 May 5. J Cell Mol Med. 2021. PMID: 33951289 Free PMC article.
-
Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn.PLoS One. 2012;7(2):e30842. doi: 10.1371/journal.pone.0030842. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363499 Free PMC article.
-
Mesenchymal stromal cells for ocular surface repair.Expert Opin Biol Ther. 2019 Jul;19(7):643-653. doi: 10.1080/14712598.2019.1607836. Epub 2019 Apr 25. Expert Opin Biol Ther. 2019. PMID: 30979344 Review.
-
Molecular and Cellular Mechanisms of the Therapeutic Effect of Mesenchymal Stem Cells and Extracellular Vesicles in Corneal Regeneration.Int J Mol Sci. 2024 Oct 16;25(20):11121. doi: 10.3390/ijms252011121. Int J Mol Sci. 2024. PMID: 39456906 Free PMC article. Review.
Cited by
-
Recent Advances in Hydrogel Technology in Delivering Mesenchymal Stem Cell for Osteoarthritis Therapy.Biomolecules. 2024 Jul 17;14(7):858. doi: 10.3390/biom14070858. Biomolecules. 2024. PMID: 39062572 Free PMC article. Review.
-
Clinical outcomes of modified simple limbal epithelial transplantation for limbal stem cell deficiency in Chinese population: a retrospective case series.Stem Cell Res Ther. 2021 May 1;12(1):259. doi: 10.1186/s13287-021-02345-2. Stem Cell Res Ther. 2021. PMID: 33933149 Free PMC article.
-
Insights and future directions for the application of perinatal derivatives in eye diseases: A critical review of preclinical and clinical studies.Front Bioeng Biotechnol. 2022 Nov 8;10:969927. doi: 10.3389/fbioe.2022.969927. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36425647 Free PMC article. Review.
-
Evaluation of colonic anastomosis healing using hybrid nanosheets containing molybdenum disulfide (MOS2) scaffold of human placental amniotic membrane and polycaprolactone (PCL) in rat animal model.Naunyn Schmiedebergs Arch Pharmacol. 2023 Sep;396(9):1911-1921. doi: 10.1007/s00210-023-02438-0. Epub 2023 Mar 2. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36859536
-
Treatment of Corneal Alkali Burn with Chestnut Honey, Royal Jelly, and Chestnut Honey-Royal Jelly Mixture.Beyoglu Eye J. 2019 Dec 27;4(3):196-201. doi: 10.14744/bej.2019.29290. eCollection 2019. Beyoglu Eye J. 2019. PMID: 35187458 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

