TRAIL (CD253) Sensitizes Human Airway Epithelial Cells to Toxin-Induced Cell Death
- PMID: 30258037
- PMCID: PMC6158510
- DOI: 10.1128/mSphere.00399-18
TRAIL (CD253) Sensitizes Human Airway Epithelial Cells to Toxin-Induced Cell Death
Abstract
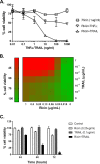
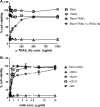
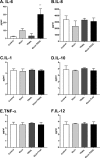
Inhalation of ricin toxin is associated with the onset of acute respiratory distress syndrome (ARDS), characterized by hemorrhage, inflammatory exudates, and tissue edema, as well as the nearly complete destruction of the lung epithelium. Here we report that the Calu-3 human airway epithelial cell line is relatively impervious to the effects of ricin, with little evidence of cell death even upon exposure to microgram amounts of toxin. However, the addition of exogenous soluble
Keywords: bioterrorism; cytokines; epithelial cells; inflammation; lung defense; toxins.
Copyright © 2018 Rong et al.
Figures


Similar articles
-
TNF Family Cytokines Induce Distinct Cell Death Modalities in the A549 Human Lung Epithelial Cell Line when Administered in Combination with Ricin Toxin.Toxins (Basel). 2019 Aug 1;11(8):450. doi: 10.3390/toxins11080450. Toxins (Basel). 2019. PMID: 31374990 Free PMC article.
-
Differential ER stress as a driver of cell fate following ricin toxin exposure.FASEB Bioadv. 2021 Oct 19;4(1):60-75. doi: 10.1096/fba.2021-00005. eCollection 2022 Jan. FASEB Bioadv. 2021. PMID: 35024573 Free PMC article.
-
Necroptosis of Lung Epithelial Cells Triggered by Ricin Toxin and Bystander Inflammation.Cell Physiol Biochem. 2023 Jan 25;57(1):1-14. doi: 10.33594/000000601. Cell Physiol Biochem. 2023. PMID: 36695077
-
Treatments for Pulmonary Ricin Intoxication: Current Aspects and Future Prospects.Toxins (Basel). 2017 Oct 3;9(10):311. doi: 10.3390/toxins9100311. Toxins (Basel). 2017. PMID: 28972558 Free PMC article. Review.
-
Divergent Roles for TRAIL in Lung Diseases.Front Med (Lausanne). 2018 Jul 27;5:212. doi: 10.3389/fmed.2018.00212. eCollection 2018. Front Med (Lausanne). 2018. PMID: 30101145 Free PMC article. Review.
Cited by
-
Passive immunization with an extended half-life monoclonal antibody protects Rhesus macaques against aerosolized ricin toxin.NPJ Vaccines. 2020 Feb 13;5(1):13. doi: 10.1038/s41541-020-0162-0. eCollection 2020. NPJ Vaccines. 2020. PMID: 32128254 Free PMC article.
-
Serum antibody profiling identifies vaccine-induced correlates of protection against aerosolized ricin toxin in rhesus macaques.NPJ Vaccines. 2022 Dec 16;7(1):164. doi: 10.1038/s41541-022-00582-x. NPJ Vaccines. 2022. PMID: 36526642 Free PMC article.
-
TNF Family Cytokines Induce Distinct Cell Death Modalities in the A549 Human Lung Epithelial Cell Line when Administered in Combination with Ricin Toxin.Toxins (Basel). 2019 Aug 1;11(8):450. doi: 10.3390/toxins11080450. Toxins (Basel). 2019. PMID: 31374990 Free PMC article.
-
A Humanized Monoclonal Antibody Cocktail to Prevent Pulmonary Ricin Intoxication.Toxins (Basel). 2020 Mar 29;12(4):215. doi: 10.3390/toxins12040215. Toxins (Basel). 2020. PMID: 32235318 Free PMC article.
-
Differential ER stress as a driver of cell fate following ricin toxin exposure.FASEB Bioadv. 2021 Oct 19;4(1):60-75. doi: 10.1096/fba.2021-00005. eCollection 2022 Jan. FASEB Bioadv. 2021. PMID: 35024573 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

