Expression of Human Cytomegalovirus IE1 Leads to Accumulation of Mono-SUMOylated PML That Is Protected from Degradation by Herpes Simplex Virus 1 ICP0
- PMID: 30258013
- PMCID: PMC6232464
- DOI: 10.1128/JVI.01452-18
Expression of Human Cytomegalovirus IE1 Leads to Accumulation of Mono-SUMOylated PML That Is Protected from Degradation by Herpes Simplex Virus 1 ICP0
Abstract
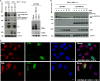
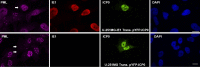
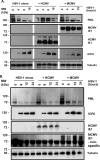
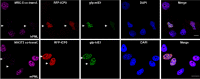
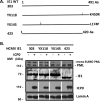
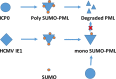
To countermeasure the host cellular intrinsic defense, cytomegalovirus (CMV) and herpes simplex viruses (HSV) have evolved the ability to disperse nuclear domain 10 (ND10, aka PML body). However, mechanisms underlying their action on ND10 differ. HSV infection produces ICP0, which degrades the ND10-forming protein PML. Human CMV (HCMV) infection expresses IE1 that deSUMOylates PML to result in dispersion of ND10. It has been demonstrated that HSV ICP0 degraded only the SUMOylated PML, so we hypothesized that HCMV IE1 can protect PML from degradation by ICP0. HCMV IE1-expressing cell lines (U-251 MG-IE1 and HELF-IE1) were used for infection of HSV-1 or transfection of ICP0-expressing plasmid. Multilabeling by immunocytochemistry assay and protein examination by Western blot assay were performed to determine the resultant fate of PML caused by ICP0 in the presence or absence of HCMV IE1. Here, we report that deSUMOylation of human PML (hPML) by HCMV IE1 was incomplete, as mono-SUMOylated PML remained in the IE1-expressing cells, which is consistent with the report by E. M. Schilling, M. Scherer, N. Reuter, J. Schweininger, et al. (J Virol 91:e02049-16, 2017, https://doi.org/10.1128/JVI.02049-16). As expected, we found that IE1 protected PML from degradation by ICP0 or HSV-1 infection. An in vitro study found that IE1 with mutation of L174P failed to deSUMOylate PML and did not protect PML from degradation by ICP0; hence, we conclude that the deSUMOylation of PML is important for IE1 to protect PML from degradation by ICP0. In addition, we revealed that murine CMV failed to deSUMOylate and to protect the HSV-mediated degradation of hPML, and that HCMV failed to deSUMOylate and protect the HSV-mediated degradation of mouse PML. However, IE1-expressing cells did not enhance wild-type HSV-1 replication but significantly increased ICP0-defective HSV-1 replication at a low multiplicity of infection. Therefore, our results uncovered a host-virus functional interaction at the posttranslational level.IMPORTANCE Our finding that HCMV IE1 protected hPML from degradation by HSV ICP0 is important, because the PML body (aka ND10) is believed to be the first line of host intrinsic defense against herpesviral infection. How the infected viruses overcome the nuclear defensive structure (PML body) has not been fully understood. Herpesviral proteins, ICP0 of HSV and IE1 of CMV, have been identified to interact with PML. Here, we report that HCMV IE1 incompletely deSUMOylated PML, resulting in the mono-SUMOylated PML, which is consistent with the report of Schilling et al. (J Virol 91:e02049-16, 2017, https://doi.org/10.1128/JVI.02049-16). The mono-SUMOylated PML was subjected to degradation by HSV ICP0. However, it was protected by IE1 from degradation by ICP0 or HSV-1 infection. In contrast, IE1 with L174P mutation lost the function of deSUMOylating PML and failed to protect the degradation of the mono-SUMOylated PML. Whether the mono-SUMOylated PML has any defensive function against viral infection will be further investigated.
Keywords: HSV-1; SUMOylation; cytomegalovirus (CMV); herpes simplex virus (HSV); immediate-early protein 1 (IE1); infected cellular protein (ICP0); nuclear domain 10 (ND10); promyelocytic leukemia protein (PML).
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Analysis of the functional interchange between the IE1 and pp71 proteins of human cytomegalovirus and ICP0 of herpes simplex virus 1.J Virol. 2015 Mar;89(6):3062-75. doi: 10.1128/JVI.03480-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552717 Free PMC article.
-
A Tale of Two PMLs: Elements Regulating a Differential Substrate Recognition by the ICP0 E3 Ubiquitin Ligase of Herpes Simplex Virus 1.J Virol. 2016 Nov 14;90(23):10875-10885. doi: 10.1128/JVI.01636-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27681131 Free PMC article.
-
Effect of SUMO-SIM Interaction on the ICP0-Mediated Degradation of PML Isoform II and Its Associated Proteins in Herpes Simplex Virus 1 Infection.J Virol. 2020 Jun 1;94(12):e00470-20. doi: 10.1128/JVI.00470-20. Print 2020 Jun 1. J Virol. 2020. PMID: 32295906 Free PMC article.
-
The potential link between PML NBs and ICP0 in regulating lytic and latent infection of HSV-1.Protein Cell. 2012 May;3(5):372-82. doi: 10.1007/s13238-012-2021-x. Epub 2012 Apr 28. Protein Cell. 2012. PMID: 22544561 Free PMC article. Review.
-
The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection.Virus Res. 2020 Aug;285:198015. doi: 10.1016/j.virusres.2020.198015. Epub 2020 May 13. Virus Res. 2020. PMID: 32416261 Free PMC article. Review.
Cited by
-
Bright and Early: Inhibiting Human Cytomegalovirus by Targeting Major Immediate-Early Gene Expression or Protein Function.Viruses. 2020 Jan 16;12(1):110. doi: 10.3390/v12010110. Viruses. 2020. PMID: 31963209 Free PMC article. Review.
-
Revisiting promyelocytic leukemia protein targeting by human cytomegalovirus immediate-early protein 1.PLoS Pathog. 2020 May 4;16(5):e1008537. doi: 10.1371/journal.ppat.1008537. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32365141 Free PMC article.
References
-
- Fagioli M, Alcalay M, Pandolfi PP, Venturini L, Mencarelli A, Simeone A, Acampora D, Grignani F, Pelicci PG. 1992. Alternative splicing of PML transcripts predicts coexpression of several carboxy-terminally different protein isoforms. Oncogene 7:1083–1091. - PubMed
-
- Borrow J, Goddard AD, Gibbons B, Katz F, Swirsky D, Fioretos T, Dube I, Winfield DA, Kingston J, Hagemeijer A. 1992. Diagnosis of acute promyelocytic leukaemia by RT-PCR: detection of PML-RARA and RARA-PML fusion transcripts. Br J Haematol 82:529–540. doi:10.1111/j.1365-2141.1992.tb06463.x. - DOI - PubMed
-
- Goddard AD, Borrow J, Solomon E. 1992. A previously uncharacterized gene, PML, is fused to the retinoic acid receptor alpha gene in acute promyelocytic leukaemia. Leukemia 6:117S–119S. - PubMed
-
- Kakizuka A, Miller WH Jr, Umesono K, Warrell RP Jr, Frankel SR, Murty VV, Dmitrovsky E, Evans RM. 1991. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 66:663–674. doi:10.1016/0092-8674(91)90112-C. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

