Indoximod: An Immunometabolic Adjuvant That Empowers T Cell Activity in Cancer
- PMID: 30254983
- PMCID: PMC6141803
- DOI: 10.3389/fonc.2018.00370
Indoximod: An Immunometabolic Adjuvant That Empowers T Cell Activity in Cancer
Abstract
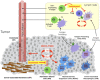
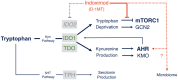
Exploding interest in immunometabolism as a source of new cancer therapeutics has been driven in large part by studies of tryptophan catabolism mediated by IDO/TDO enzymes. A chief focus in the field is IDO1, a pro-inflammatory modifier that is widely overexpressed in cancers where it blunts immunosurveillance and enables neovascularization and metastasis. The simple racemic compound 1-methyl-D,L-tryptophan (1MT) is an extensively used probe of IDO/TDO pathways that exerts a variety of complex inhibitory effects. The L isomer of 1MT is a weak substrate for IDO1 and is ascribed the weak inhibitory activity of the racemate on the enzyme. In contrast, the D isomer neither binds nor inhibits the purified IDO1 enzyme. However, clinical development focused on D-1MT (now termed indoximod) due to preclinical cues of its greater anticancer activity and its distinct mechanisms of action. In contrast to direct enzymatic inhibitors of IDO1, indoximod acts downstream of IDO1 to stimulate mTORC1, a convergent effector signaling molecule for all IDO/TDO enzymes, thus possibly lowering risks of drug resistance by IDO1 bypass. In this review, we survey the unique biological and mechanistic features of indoximod as an IDO/TDO pathway inhibitor, including recent clinical findings of its ability to safely enhance various types of cancer therapy, including chemotherapy, chemo-radiotherapy, vaccines, and immune checkpoint therapy. We also review the potential advantages indoximod offers compared to selective IDO1-specific blockade, which preclinical studies and the clinical study ECHO-301 suggest may be bypassed readily by tumors. Indoximod lies at a leading edge of broad-spectrum immunometabolic agents that may act to improve responses to many anticancer modalities, in a manner analogous to vaccine adjuvants that act to boost immunity in settings of infectious disease.
Keywords: Immunotherapy; immune adjuvant; immuno-chemotherapy; immuno-radiotherapy; immunometabolism.
Figures
Similar articles
-
Indoximod opposes the immunosuppressive effects mediated by IDO and TDO via modulation of AhR function and activation of mTORC1.Oncotarget. 2020 Jun 23;11(25):2438-2461. doi: 10.18632/oncotarget.27646. eCollection 2020 Jun 23. Oncotarget. 2020. PMID: 32637034 Free PMC article.
-
Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer.Int Rev Cell Mol Biol. 2018;336:175-203. doi: 10.1016/bs.ircmb.2017.07.004. Epub 2017 Sep 21. Int Rev Cell Mol Biol. 2018. PMID: 29413890 Free PMC article. Review.
-
Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond.Semin Immunopathol. 2019 Jan;41(1):41-48. doi: 10.1007/s00281-018-0702-0. Epub 2018 Sep 10. Semin Immunopathol. 2019. PMID: 30203227 Review.
-
Inflammatory Reprogramming with IDO1 Inhibitors: Turning Immunologically Unresponsive 'Cold' Tumors 'Hot'.Trends Cancer. 2018 Jan;4(1):38-58. doi: 10.1016/j.trecan.2017.11.005. Epub 2017 Dec 21. Trends Cancer. 2018. PMID: 29413421 Free PMC article. Review.
-
Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO.Front Immunol. 2021 Feb 23;12:636081. doi: 10.3389/fimmu.2021.636081. eCollection 2021. Front Immunol. 2021. PMID: 33708223 Free PMC article. Review.
Cited by
-
The Immunomodulator 1-Methyltryptophan Drives Tryptophan Catabolism Toward the Kynurenic Acid Branch.Front Immunol. 2020 Feb 28;11:313. doi: 10.3389/fimmu.2020.00313. eCollection 2020. Front Immunol. 2020. PMID: 32180772 Free PMC article.
-
Indoximod opposes the immunosuppressive effects mediated by IDO and TDO via modulation of AhR function and activation of mTORC1.Oncotarget. 2020 Jun 23;11(25):2438-2461. doi: 10.18632/oncotarget.27646. eCollection 2020 Jun 23. Oncotarget. 2020. PMID: 32637034 Free PMC article.
-
Increased effect of two-fraction radiotherapy in conjunction with IDO1 inhibition in experimental glioblastoma.PLoS One. 2020 May 29;15(5):e0233617. doi: 10.1371/journal.pone.0233617. eCollection 2020. PLoS One. 2020. PMID: 32469935 Free PMC article.
-
The Evolving Role of Radiotherapy for Pediatric Cancers With Advancements in Molecular Tumor Characterization and Targeted Therapies.Front Oncol. 2021 Sep 16;11:679701. doi: 10.3389/fonc.2021.679701. eCollection 2021. Front Oncol. 2021. PMID: 34604027 Free PMC article. Review.
-
Nanodelivery Optimization of IDO1 Inhibitors in Tumor Immunotherapy: Challenges and Strategies.Int J Nanomedicine. 2024 Aug 28;19:8847-8882. doi: 10.2147/IJN.S458086. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39220190 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

