A requirement for Fgfr2 in middle ear development
- PMID: 30253032
- PMCID: PMC6349551
- DOI: 10.1002/dvg.23252
A requirement for Fgfr2 in middle ear development
Abstract
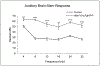
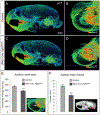
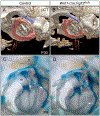
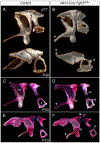
The skeletal structure of the mammalian middle ear, which is composed of three endochondral ossicles suspended within a membranous air-filled capsule, plays a critical role in conducting sound. Gene mutations that alter skeletal development in the middle ear result in auditory impairment. Mutations in fibroblast growth factor receptor 2 (FGFR2), an important regulator of endochondral and intramembranous bone formation, cause a spectrum of congenital skeletal disorders featuring conductive hearing loss. Although the middle ear malformations in multiple FGFR2 gain-of-function disorders are clinically characterized, those in the FGFR2 loss-of-function disorder lacrimo-auriculo-dento-digital (LADD) syndrome are relatively undescribed. To better understand conductive hearing loss in LADD, we examined the middle ear skeleton of mice with conditional loss of Fgfr2. We find that decreased auditory function in Fgfr2 mutant mice correlates with hypoplasia of the auditory bulla and ectopic bone growth at sites of tendon/ligament attachment. We show that ectopic bone associated with the intra-articular ligaments of the incudomalleal joint is derived from Scx-expressing cells and preceded by decreased expression of the joint progenitor marker Gdf5. Together, these results identify a role for Fgfr2 in development of the middle ear skeletal tissues and suggest potential causes for conductive hearing loss in LADD syndrome.
Keywords: Fgfr2; auditory ossicles; craniofacial development; joint development; skeletal development.
© 2018 Wiley Periodicals, Inc.
Figures



Similar articles
-
Lacrimo-auriculo-dento-digital syndrome: A novel mutation in a Korean family and review of literature.Mol Genet Genomic Med. 2020 Oct;8(10):e1412. doi: 10.1002/mgg3.1412. Epub 2020 Jul 26. Mol Genet Genomic Med. 2020. PMID: 32715658 Free PMC article. Review.
-
LADD syndrome with glaucoma is caused by a novel gene.Mol Vis. 2017 Mar 30;23:179-184. eCollection 2017. Mol Vis. 2017. PMID: 28400699 Free PMC article.
-
Identification of a novel missence mutation in FGFR3 gene in an Iranian family with LADD syndrome by Next-Generation Sequencing.Int J Pediatr Otorhinolaryngol. 2017 Jun;97:192-196. doi: 10.1016/j.ijporl.2017.04.016. Epub 2017 Apr 12. Int J Pediatr Otorhinolaryngol. 2017. PMID: 28483234 Review.
-
[Clinical diagnosis of familial Levy-Hollister syndrome].An Pediatr (Barc). 2014 Feb;80(2):114-6. doi: 10.1016/j.anpedi.2013.02.019. Epub 2013 Apr 2. An Pediatr (Barc). 2014. PMID: 23562527 Spanish.
-
Dental issues in lacrimo-auriculo-dento-digital syndrome: An autosomal dominant condition with clinical and genetic variability.J Am Dent Assoc. 2017 Mar;148(3):157-163. doi: 10.1016/j.adaj.2016.11.016. Epub 2016 Dec 30. J Am Dent Assoc. 2017. PMID: 28043400
Cited by
-
New developments in the biology of fibroblast growth factors.WIREs Mech Dis. 2022 Jul;14(4):e1549. doi: 10.1002/wsbm.1549. Epub 2022 Feb 9. WIREs Mech Dis. 2022. PMID: 35142107 Free PMC article. Review.
-
The impact of Drew Noden's work on our understanding of craniofacial musculoskeletal integration.Dev Dyn. 2022 Aug;251(8):1250-1266. doi: 10.1002/dvdy.471. Epub 2022 Apr 5. Dev Dyn. 2022. PMID: 35338756 Free PMC article. Review.
-
Uncovering the secreted signals and transcription factors regulating the development of mammalian middle ear ossicles.Dev Dyn. 2020 Dec;249(12):1410-1424. doi: 10.1002/dvdy.260. Epub 2020 Oct 28. Dev Dyn. 2020. PMID: 33058336 Free PMC article. Review.
References
-
- Aimi K 1983. Role of the tympanic ring in the pathogenesis of congenital cholesteatoma. Laryngoscope 93: 1140–1146. - PubMed
-
- Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bosl MR, Kato S, Maconochie M, Riethmacher D, Schimmang T. 2003. Requirements for FGF3 and FGF10 during inner ear formation. Development 130: 6329–6338. - PubMed
-
- Amin S, Tucker AS. 2006. Joint formation in the middle ear: lessons from the mouse and guinea pig. Dev Dyn 235: 1326–1333. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

