Extracellular matrix with defective collagen cross-linking affects the differentiation of bone cells
- PMID: 30252876
- PMCID: PMC6155528
- DOI: 10.1371/journal.pone.0204306
Extracellular matrix with defective collagen cross-linking affects the differentiation of bone cells
Abstract
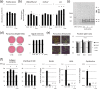
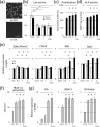
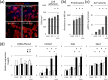
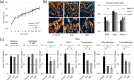
Fibrillar type I collagen, the predominant organic component in bone, is stabilized by lysyl oxidase (LOX)-initiated covalent intermolecular cross-linking, an important determinant of bone quality. However, the impact of collagen cross-linking on the activity of bone cells and subsequent tissue remodeling is not well understood. In this study, we investigated the effect of collagen cross-linking on bone cellular activities employing a loss-of-function approach, using a potent LOX inhibitor, β-aminopropionitrile (BAPN). Osteoblastic cells (MC3T3-E1) were cultured for 2 weeks in the presence of 0-2 mM BAPN to obtain low cross-linked collagen matrices. The addition of BAPN to the cultures diminished collagen cross-links in a dose-dependent manner and, at 1 mM level, none of the major cross-links were detected without affecting collagen production. After the removal of cellular components from these cultures, MC3T3-E1, osteoclasts (RAW264.7), or mouse primary bone marrow-derived stromal cells (BMSCs) were seeded. MC3T3-E1 cells grown on low cross-link matrices showed increased alkaline phosphatase (ALP) activity. The number of multinucleate tartrate-resistant acid phosphatase (TRAP)-positive cells increased in RAW264.7 cells. Initial adhesion, proliferation, and ALP activity of BMSCs also increased. In the animal experiments, 4-week-old C57BL/6 mice were fed with BAPN-containing diet for 8 weeks. At this point, biochemical analysis of bone demonstrated that collagen cross-links decreased without affecting collagen content. Then, the diet was changed to a control diet to minimize the direct effect of BAPN. At 2 and 4 weeks after the change, histological samples were prepared. Histological examination of femur samples at 4 weeks showed a significant increase in the number of bone surface osteoblasts, while the bone volume and surface osteoclast numbers were not significantly affected. These results clearly demonstrated that the extent of collagen cross-linking of bone matrix affected the differentiation of bone cells, underscoring the importance of collagen cross-linking in the regulation of cell behaviors and tissue remodeling in bone. Characterization of collagen cross-linking in bone may be beneficial to obtain insight into not only bone mechanical property, but also bone cellular activities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Collagen cross-linking influences osteoblastic differentiation.Calcif Tissue Int. 2008 May;82(5):392-400. doi: 10.1007/s00223-008-9136-3. Epub 2008 May 17. Calcif Tissue Int. 2008. PMID: 18488133
-
Differential effects of homocysteine and beta aminopropionitrile on preosteoblastic MC3T3-E1 cells.Bone. 2010 Mar;46(3):703-9. doi: 10.1016/j.bone.2009.10.038. Epub 2009 Nov 4. Bone. 2010. PMID: 19895920
-
The Effect of β-Aminopropionitrile on Skeletal Micromorphology and Osteogenesis.Calcif Tissue Int. 2018 Oct;103(4):411-421. doi: 10.1007/s00223-018-0430-4. Epub 2018 Jun 18. Calcif Tissue Int. 2018. PMID: 29916126
-
Icariin influences adipogenic differentiation of stem cells affected by osteoblast-osteoclast co-culture and clinical research adipogenic.Biomed Pharmacother. 2017 Apr;88:436-442. doi: 10.1016/j.biopha.2017.01.050. Epub 2017 Jan 22. Biomed Pharmacother. 2017. PMID: 28122309
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
Cited by
-
Switch of macrophage fusion competency by 3D matrices.Sci Rep. 2020 Jun 25;10(1):10348. doi: 10.1038/s41598-020-67056-9. Sci Rep. 2020. PMID: 32587271 Free PMC article.
-
Fibrosis in Mesothelioma: Potential Role of Lysyl Oxidases.Cancers (Basel). 2022 Feb 15;14(4):981. doi: 10.3390/cancers14040981. Cancers (Basel). 2022. PMID: 35205728 Free PMC article. Review.
-
The effect of caponization on tibia bone histomorphometric properties of crossbred roosters.Sci Rep. 2024 Feb 19;14(1):4062. doi: 10.1038/s41598-024-54791-6. Sci Rep. 2024. PMID: 38374163 Free PMC article.
-
Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature.J Endocr Soc. 2019 Jul 10;3(10):1799-1818. doi: 10.1210/js.2019-00160. eCollection 2019 Oct 1. J Endocr Soc. 2019. PMID: 31528827 Free PMC article. Review.
-
Degradation of subchondral bone collagen in the weight-bearing area of femoral head is associated with osteoarthritis and osteonecrosis.J Orthop Surg Res. 2020 Nov 11;15(1):526. doi: 10.1186/s13018-020-02065-y. J Orthop Surg Res. 2020. PMID: 33176818 Free PMC article.
References
-
- Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22(3):181–7. . - PubMed
-
- Shiiba M, Arnaud SB, Tanzawa H, Kitamura E, Yamauchi M. Regional alterations of type I collagen in rat tibia induced by skeletal unloading. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002;17(9):1639–45. 10.1359/jbmr.2002.17.9.1639 . - DOI - PubMed
-
- Shiiba M, Arnaud SB, Tanzawa H, Uzawa K, Yamauchi M. Alterations of collagen matrix in weight-bearing bones during skeletal unloading. Connective tissue research. 2001;42(4):303–11. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

