CD8+ T cells modulate autosomal dominant polycystic kidney disease progression
- PMID: 30249452
- PMCID: PMC6319903
- DOI: 10.1016/j.kint.2018.06.025
CD8+ T cells modulate autosomal dominant polycystic kidney disease progression
Abstract
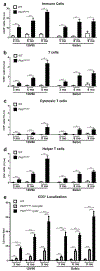
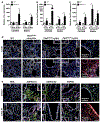
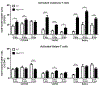
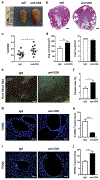
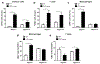
Autosomal dominant polycystic kidney disease (ADPKD) is the most prevalent inherited nephropathy. To date, therapies alleviating the disease have largely focused on targeting abnormalities in renal epithelial cell signaling. ADPKD has many hallmarks of cancer, where targeting T cells has brought novel therapeutic interventions. However, little is known about the role and therapeutic potential of T cells in ADPKD. Here, we used an orthologous ADPKD model, Pkd1 p.R3277C (RC), to begin to define the role of T cells in disease progression. Using flow cytometry, we found progressive increases in renal CD8+ and CD4+ T cells, correlative with disease severity, but with selective activation of CD8+ T cells. By immunofluorescence, T cells specifically localized to cystic lesions and increased levels of T-cell recruiting chemokines (CXCL9/CXCL10) were detected by qPCR/in situ hybridization in the kidneys of mice, patients, and ADPKD epithelial cell lines. Importantly, immunodepletion of CD8+ T cells from one to three months in C57Bl/6 Pkd1RC/RC mice resulted in worsening of ADPKD pathology, decreased apoptosis, and increased proliferation compared to IgG-control, consistent with a reno-protective role of CD8+ T cells. Thus, our studies suggest a functional role for T cells, specifically CD8+ T cells, in ADPKD progression. Hence, targeting this pathway using immune-oncology agents may represent a novel therapeutic approach for ADPKD.
Keywords: CD8(+) T cells; Pkd1 RC mouse model; adaptive immunity; autosomal dominant polycystic kidney disease.
Copyright © 2018 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
All the authors declared no competing interests.
Figures
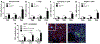
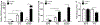
Similar articles
-
Metabolic reprogramming in a slowly developing orthologous model of polycystic kidney disease.Am J Physiol Renal Physiol. 2022 Mar 1;322(3):F258-F267. doi: 10.1152/ajprenal.00262.2021. Epub 2022 Jan 17. Am J Physiol Renal Physiol. 2022. PMID: 35037466 Free PMC article.
-
Metformin improves relevant disease parameters in an autosomal dominant polycystic kidney disease mouse model.Am J Physiol Renal Physiol. 2022 Jan 1;322(1):F27-F41. doi: 10.1152/ajprenal.00298.2021. Epub 2021 Nov 22. Am J Physiol Renal Physiol. 2022. PMID: 34806449
-
RNA helicase p68 inhibits the transcription and post-transcription of Pkd1 in ADPKD.Theranostics. 2020 Jul 9;10(18):8281-8297. doi: 10.7150/thno.47315. eCollection 2020. Theranostics. 2020. PMID: 32724471 Free PMC article.
-
Perspectives of Gene Therapies in Autosomal Dominant Polycystic Kidney Disease.Curr Gene Ther. 2017;17(1):43-49. doi: 10.2174/1566523217666170510152808. Curr Gene Ther. 2017. PMID: 28494735 Review.
-
Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD).Biochim Biophys Acta. 2011 Oct;1812(10):1327-36. doi: 10.1016/j.bbadis.2011.06.012. Epub 2011 Jul 1. Biochim Biophys Acta. 2011. PMID: 21745567 Free PMC article. Review.
Cited by
-
The tryptophan-metabolizing enzyme indoleamine 2,3-dioxygenase 1 regulates polycystic kidney disease progression.JCI Insight. 2023 Jan 10;8(1):e154773. doi: 10.1172/jci.insight.154773. JCI Insight. 2023. PMID: 36422996 Free PMC article.
-
Cluster of differentiation 8 and programmed cell death ligand 1 expression in triple-negative breast cancer combined with autosomal dominant polycystic kidney disease and tuberous sclerosis complex: a case report.J Med Case Rep. 2019 Dec 24;13(1):381. doi: 10.1186/s13256-019-2274-6. J Med Case Rep. 2019. PMID: 31870441 Free PMC article.
-
Role of chemokines, innate and adaptive immunity.Cell Signal. 2020 Sep;73:109647. doi: 10.1016/j.cellsig.2020.109647. Epub 2020 Apr 20. Cell Signal. 2020. PMID: 32325183 Free PMC article. Review.
-
Myofibroblast depletion reduces kidney cyst growth and fibrosis in autosomal dominant polycystic kidney disease.Kidney Int. 2023 Jan;103(1):144-155. doi: 10.1016/j.kint.2022.08.036. Epub 2022 Oct 20. Kidney Int. 2023. PMID: 36273656 Free PMC article.
-
Metabolic reprogramming in a slowly developing orthologous model of polycystic kidney disease.Am J Physiol Renal Physiol. 2022 Mar 1;322(3):F258-F267. doi: 10.1152/ajprenal.00262.2021. Epub 2022 Jan 17. Am J Physiol Renal Physiol. 2022. PMID: 35037466 Free PMC article.
References
-
- Grantham JJ. Mechanisms of progression in autosomal dominant polycystic kidney disease. Kidney Int Suppl. 1997;63:S93–S97. - PubMed
-
- Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1:148–157. - PubMed
-
- Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–1485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

