Evaluation of the roles of the cytosolic N-terminus and His-rich loop of ZNT proteins using ZNT2 and ZNT3 chimeric mutants
- PMID: 30237557
- PMCID: PMC6147782
- DOI: 10.1038/s41598-018-32372-8
Evaluation of the roles of the cytosolic N-terminus and His-rich loop of ZNT proteins using ZNT2 and ZNT3 chimeric mutants
Abstract
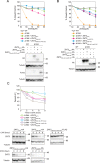
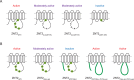
The physiological roles of Zn transporter (ZNT) proteins are being increasingly recognized, and three dimensional structures of ZNT bacterial homologs have facilitated our understanding of their biochemical characteristics at the molecular level. However, the biological role of the unique structural features of vertebrate ZNTs, which are absent in their bacterial homologues, is not completely understood. These ZNT sequences include a cytosolic His-rich loop between transmembrane helices IV and V and the cytosolic N-terminus. This study investigated the contribution of these features to zinc transport by ZNT proteins. The importance of the His residues in the cytosolic His-rich loop was investigated using ZNT2 Ala substitution and deletion mutants. The presence of His residues was not essential for zinc transport, even though they possibly participate in modulation of zinc transport activity. Furthermore, we determined the role of the N-terminus by characterizing ZNT2 and ZNT3 domain-swapped and deletion mutants. Unexpectedly, the N-terminus was also not essential for zinc transport by ZNT2 and the domain-swapped ZNT2 mutant, in which the cytosolic His-rich loop was substituted with that of ZNT3. These results provide molecular insights into understanding the roles of the cytosolic parts of ZNT2, ZNT3, and probably other members of their subgroup.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Demonstrating aspects of multiscale modeling by studying the permeation pathway of the human ZnT2 zinc transporter.PLoS Comput Biol. 2018 Nov 2;14(11):e1006503. doi: 10.1371/journal.pcbi.1006503. eCollection 2018 Nov. PLoS Comput Biol. 2018. PMID: 30388104 Free PMC article.
-
Acute phase expression pattern of ZnTs, LC3, and beclin-1 in rat Hippocampus and its regulation by 3-methyladenine following recurrent neonatal seizures.Biol Trace Elem Res. 2011 Oct;143(1):320-31. doi: 10.1007/s12011-010-8836-5. Epub 2010 Sep 14. Biol Trace Elem Res. 2011. PMID: 20838925
-
The presence and response to Zn of ZnT family mRNAs in human dental pulp.Metallomics. 2019 Mar 20;11(3):613-620. doi: 10.1039/c8mt00343b. Metallomics. 2019. PMID: 30675888
-
Molecular architecture and function of ZnT transporters.Curr Top Membr. 2012;69:199-220. doi: 10.1016/B978-0-12-394390-3.00008-2. Curr Top Membr. 2012. PMID: 23046652 Review.
-
Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function.Neurosci Biobehav Rev. 2017 Sep;80:329-350. doi: 10.1016/j.neubiorev.2017.06.006. Epub 2017 Jun 15. Neurosci Biobehav Rev. 2017. PMID: 28624432 Review.
Cited by
-
Sophisticated expression responses of ZNT1 and MT in response to changes in the expression of ZIPs.Sci Rep. 2022 May 5;12(1):7334. doi: 10.1038/s41598-022-10925-2. Sci Rep. 2022. PMID: 35513474 Free PMC article.
-
Metal transport mechanism of the cation diffusion facilitator (CDF) protein family - a structural perspective on human CDF (ZnT)-related diseases.RSC Chem Biol. 2021 Jan 25;2(2):486-498. doi: 10.1039/d0cb00181c. eCollection 2021 Apr 1. RSC Chem Biol. 2021. PMID: 34458794 Free PMC article. Review.
-
Loss of Znt8 function in diabetes mellitus: risk or benefit?Mol Cell Biochem. 2021 Jul;476(7):2703-2718. doi: 10.1007/s11010-021-04114-4. Epub 2021 Mar 5. Mol Cell Biochem. 2021. PMID: 33666829 Review.
-
Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels.J Biol Chem. 2019 Oct 25;294(43):15686-15697. doi: 10.1074/jbc.RA119.010227. Epub 2019 Aug 30. J Biol Chem. 2019. PMID: 31471319 Free PMC article.
-
Demonstrating aspects of multiscale modeling by studying the permeation pathway of the human ZnT2 zinc transporter.PLoS Comput Biol. 2018 Nov 2;14(11):e1006503. doi: 10.1371/journal.pcbi.1006503. eCollection 2018 Nov. PLoS Comput Biol. 2018. PMID: 30388104 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

