Acute molecular effects of pressure-controlled intermittent coronary sinus occlusion in patients with advanced heart failure
- PMID: 30230713
- PMCID: PMC6301157
- DOI: 10.1002/ehf2.12354
Acute molecular effects of pressure-controlled intermittent coronary sinus occlusion in patients with advanced heart failure
Abstract
Aims: Cardiac repair has steered clinical attention and remains an unmet need, because available regenerative therapies lack robust mechanistic evidence. Pressure-controlled intermittent coronary sinus occlusion (PICSO), known to induce angiogenetic and vasoactive molecules as well as to reduce regional ischemia, may activate endogenous regenerative processes in failing myocardium. We aimed to investigate the effects of PICSO in patients with advanced heart failure undergoing cardiac resynchronization therapy.
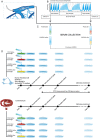
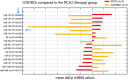
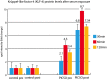
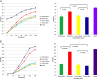
Methods and results: Eight out of 32 patients were treated with PICSO, and the remainder served as controls. After electrode testing including left ventricular leads, PICSO was performed for 20 min. To test immediate molecular responses, in both patient groups, coronary venous blood samples were taken at baseline and after 20 min, the time required for the intervention. Sera were tested for microRNAs and growth factors. To test the ability of up-regulated soluble factors on cell proliferation and expression of transcription factors [e.g. Krüppel-like factor 4 (KLF-4)], sera were co-cultured with human cardiomyocytes and fibroblasts. As compared with controls, significant differential expression (differences between pre-values and post-values in relation to both patient cohorts) of microRNA patterns associated with cardiac development was observed with PICSO. Importantly, miR-143 (P < 0.048) and miR-145 (P < 0,047) increased, both targeting a network of transcription factors (including KLF-4) that promote differentiation and repress proliferation of vascular smooth muscle cells. Additionally, an increase of miR-19b (P < 0.019) known to alleviate endothelial cell apoptosis was found, whereas disadvantageous miR-320b (P < 0.023) suspect to impair expression of c-myc, normally provoking cell cycle re-entry in post-mitotic myocytes and miR-25 (P < 0.023), decreased, a target of anti-miR application to improve contractility in the failing heart. Co-cultured post-PICSO sera significantly increased cellular proliferation both in fibroblasts (P < 0.001) and adult cardiomycytes (P < 0.004) sampled from a transplant recipient as compared with controls. Adult cardiomyocytes showed a seven-fold increase of the transcription factor KLF-4 protein when co-cultured with treated sera as compared with controls.
Conclusions: Here, we show for the first time that PICSO, a trans-coronary sinus catheter intervention, is associated with an increase in morphogens secreted into cardiac veins, normally present during cardiac development, and a significant induction of cell proliferation. Present findings support the notion that epigenetic modifications, that is, haemodynamic stimuli on venous vascular cells, may reverse myocardial deterioration. Further investigations are needed to decipher the maze of complex interacting molecular pathways in failing myocardium and the potential role of PICSO to reinitiate developmental processes to prevent further myocardial decay eventually reaching clinical significance.
Keywords: Cardiac regeneration; Embryonic recall; Heart failure; PICSO.
© 2018 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of the European Society of Cardiology.
Figures
Similar articles
-
Circulating microRNAs and cardiomyocyte proliferation in heart failure patients related to 10 years survival.ESC Heart Fail. 2023 Dec;10(6):3559-3572. doi: 10.1002/ehf2.14516. Epub 2023 Sep 26. ESC Heart Fail. 2023. PMID: 37752740 Free PMC article.
-
Pressure-controlled intermittent coronary sinus occlusion (PICSO) in acute ST-segment elevation myocardial infarction: results of the Prepare RAMSES safety and feasibility study.EuroIntervention. 2015 May;11(1):37-44. doi: 10.4244/EIJY15M03_10. EuroIntervention. 2015. PMID: 25868741 Clinical Trial.
-
PICSO: from myocardial salvage to tissue regeneration.Cardiovasc Revasc Med. 2015 Jan-Feb;16(1):36-46. doi: 10.1016/j.carrev.2014.12.004. Epub 2014 Dec 23. Cardiovasc Revasc Med. 2015. PMID: 25616738 Review.
-
Intracoronary hemodynamic effects of pressure-controlled intermittent coronary sinus occlusion (PICSO): results from the First-In-Man Prepare PICSO Study.J Interv Cardiol. 2012 Dec;25(6):549-56. doi: 10.1111/j.1540-8183.2012.00768.x. Epub 2012 Sep 20. J Interv Cardiol. 2012. PMID: 22994798
-
Coronary sinus retroperfusion and pressure-controlled intermittent coronary sinus occlusion (PICSO) for myocardial protection.Surg Clin North Am. 1985 Jun;65(3):477-95. doi: 10.1016/s0039-6109(16)43632-7. Surg Clin North Am. 1985. PMID: 3898427 Review.
Cited by
-
The Use of Cardioprotective Devices and Strategies in Patients Undergoing Percutaneous Procedures and Cardiac Surgery.Healthcare (Basel). 2023 Apr 11;11(8):1094. doi: 10.3390/healthcare11081094. Healthcare (Basel). 2023. PMID: 37107928 Free PMC article. Review.
-
Circulating microRNAs and cardiomyocyte proliferation in heart failure patients related to 10 years survival.ESC Heart Fail. 2023 Dec;10(6):3559-3572. doi: 10.1002/ehf2.14516. Epub 2023 Sep 26. ESC Heart Fail. 2023. PMID: 37752740 Free PMC article.
-
New Interventional Therapies beyond Stenting to Treat ST-Segment Elevation Acute Myocardial Infarction.J Cardiovasc Dev Dis. 2021 Aug 24;8(9):100. doi: 10.3390/jcdd8090100. J Cardiovasc Dev Dis. 2021. PMID: 34564118 Free PMC article. Review.
-
Plasma microRNA-143 and microRNA-145 levels are elevated in patients with left ventricular dysfunction.Heart Vessels. 2024 Oct;39(10):867-876. doi: 10.1007/s00380-024-02410-9. Epub 2024 May 8. Heart Vessels. 2024. PMID: 38717698 Free PMC article.
-
Effect of Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) on infarct size in anterior STEMI: PiCSO in ACS study.Int J Cardiol Heart Vasc. 2020 May 15;28:100526. doi: 10.1016/j.ijcha.2020.100526. eCollection 2020 Jun. Int J Cardiol Heart Vasc. 2020. PMID: 32435689 Free PMC article.
References
-
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. - PubMed
-
- Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisen J, Giacca M, Hare JM, Houser S, Lee RT, Marbán E, Martin JF, Molkentin JD, Murry CE, Riley PR, Ruiz‐Lozano P, Sadek HA, Sussman MA, Hill JA. Cardiomyocyte regeneration: a consensus statement. Circulation 2017; 136: 680–686. - PMC - PubMed
-
- Mohl W, Gangl C, Jusić A, Aschacher T, De Jonge M, Rattay F. PICSO: from myocardial salvage to tissue regeneration. Cardiovasc Revasc Med 2015; 16: 36–46. - PubMed
-
- Pappalardo F, Ancona MB, Giannini F, Regazzoli D, Mangieri A, Montorfano M, de Bonis M, Alfieri O, Zangrillo A, Scandroglio AM, Colombo A, Latib A. First in man prolonged pressure‐controlled intermittent coronary sinus occlusion to treat refractory left ventricular dysfunction and ischaemia with patent epicardial coronary arteries. Int J Cardiol 2017; 241: 138–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

