A Subpopulation of Foxj1-Expressing, Nonmyelinating Schwann Cells of the Peripheral Nervous System Contribute to Schwann Cell Remyelination in the Central Nervous System
- PMID: 30228229
- PMCID: PMC6199410
- DOI: 10.1523/JNEUROSCI.0585-18.2018
A Subpopulation of Foxj1-Expressing, Nonmyelinating Schwann Cells of the Peripheral Nervous System Contribute to Schwann Cell Remyelination in the Central Nervous System
Abstract
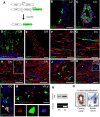
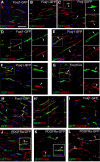
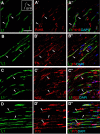
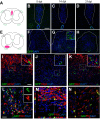
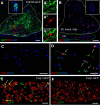
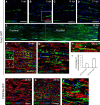
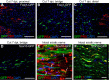
New myelin sheaths can be restored to demyelinated axons in a spontaneous regenerative process called remyelination. In general, new myelin sheaths are made by oligodendrocytes newly generated from a widespread population of adult CNS progenitors called oligodendrocyte progenitor cells (OPCs). New myelin in CNS remyelination in both experimental models and clinical diseases can also be generated by Schwann cells (SCs), the myelin-forming cells of the PNS. Fate-mapping studies have shown that SCs contributing to remyelination in the CNS are often derived from OPCs and appear not to be derived from myelinating SCs from the PNS. In this study, we address whether CNS remyelinating SCs can also be generated from PNS-derived cells other than myelinating SCs. Using a genetic fate-mapping approach, we have found that a subpopulation of nonmyelinating SCs identified by the expression of the transcription factor Foxj1 also contribute to CNS SC remyelination, as well as to remyelination in the PNS. We also find that the ependymal cells lining the central canal of the spinal cord, which also express Foxj1, do not generate cells that contribute to CNS remyelination. These findings therefore identify a previously unrecognized population of PNS glia that can participate in the regeneration of new myelin sheaths following CNS demyelination.SIGNIFICANCE STATEMENT Remyelination failure in chronic demyelinating diseases such as multiple sclerosis drives the current quest for developing means by which remyelination in CNS can be enhanced therapeutically. Critical to this endeavor is the need to understand the mechanisms of remyelination, including the nature and identity of the cells capable of generating new myelin sheath-forming cells. Here, we report a previously unrecognized subpopulation of nonmyelinating Schwann cells (SCs) in the PNS, identified by the expression of the transcription factor Foxj1, which can give rise to SCs that are capable of remyelinating both PNS and CNS axons. These cells therefore represent a new cellular target for myelin regenerative strategies for the treatment of CNS disorders characterized by persistent demyelination.
Keywords: CNS remyelination; Foxj1; Schwann cells; peripheral nerve.
Copyright © 2018 Ma, Wang et al.
Figures

Similar articles
-
Schwann cell remyelination of the central nervous system: why does it happen and what are the benefits?Open Biol. 2021 Jan;11(1):200352. doi: 10.1098/rsob.200352. Epub 2021 Jan 27. Open Biol. 2021. PMID: 33497588 Free PMC article. Review.
-
Schwann cells: Rescuers of central demyelination.Glia. 2020 Oct;68(10):1945-1956. doi: 10.1002/glia.23788. Epub 2020 Feb 6. Glia. 2020. PMID: 32027054 Review.
-
Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin.Proc Natl Acad Sci U S A. 2016 Dec 20;113(51):14829-14834. doi: 10.1073/pnas.1614826113. Epub 2016 Dec 7. Proc Natl Acad Sci U S A. 2016. PMID: 27930320 Free PMC article.
-
Astrocyte Activation via Stat3 Signaling Determines the Balance of Oligodendrocyte versus Schwann Cell Remyelination.Am J Pathol. 2015 Sep;185(9):2431-40. doi: 10.1016/j.ajpath.2015.05.011. Epub 2015 Jul 17. Am J Pathol. 2015. PMID: 26193667 Free PMC article.
-
Study of Myelin Gene Expression in the Central Nervous System Using Real-Time PCR.Methods Mol Biol. 2019;2011:659-670. doi: 10.1007/978-1-4939-9554-7_38. Methods Mol Biol. 2019. PMID: 31273727
Cited by
-
Blood vessels guide Schwann cell migration in the adult demyelinated CNS through Eph/ephrin signaling.Acta Neuropathol. 2019 Sep;138(3):457-476. doi: 10.1007/s00401-019-02011-1. Epub 2019 Apr 22. Acta Neuropathol. 2019. PMID: 31011859 Free PMC article.
-
Wrapping glia regulates neuronal signaling speed and precision in the peripheral nervous system of Drosophila.Nat Commun. 2020 Sep 8;11(1):4491. doi: 10.1038/s41467-020-18291-1. Nat Commun. 2020. PMID: 32901033 Free PMC article.
-
Forced Remyelination Promotes Axon Regeneration in a Rat Model of Spinal Cord Injury.Int J Mol Sci. 2022 Dec 28;24(1):495. doi: 10.3390/ijms24010495. Int J Mol Sci. 2022. PMID: 36613945 Free PMC article.
-
Biomaterial-Based Schwann Cell Transplantation and Schwann Cell-Derived Biomaterials for Nerve Regeneration.Front Cell Neurosci. 2022 Jun 28;16:926222. doi: 10.3389/fncel.2022.926222. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35836742 Free PMC article. Review.
-
Transcriptional profiling of mouse peripheral nerves to the single-cell level to build a sciatic nerve ATlas (SNAT).Elife. 2021 Apr 23;10:e58591. doi: 10.7554/eLife.58591. Elife. 2021. PMID: 33890853 Free PMC article.
References
-
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) c-jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633–647. 10.1016/j.neuron.2012.06.021 - DOI - PMC - PubMed
-
- Assinck P, Duncan GJ, Plemel JR, Lee MJ, Stratton JA, Manesh SB, Liu J, Ramer LM, Kang SH, Bergles DE, Biernaskie J, Tetzlaff W (2017) Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J Neurosci 37:8635–8654. 10.1523/JNEUROSCI.2409-16.2017 - DOI - PMC - PubMed
-
- Carlén M, Meletis K, Göritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabé-Heider F, Yeung MS, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, **Frisén J (2009) Forebrain ependymal cells are notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci 12:259–267. 10.1038/nn.2268 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
