Distinct phenotypes and 'bystander' effects of senescent tumour cells induced by docetaxel or immunomodulatory cytokines
- PMID: 30226595
- PMCID: PMC6192732
- DOI: 10.3892/ijo.2018.4553
Distinct phenotypes and 'bystander' effects of senescent tumour cells induced by docetaxel or immunomodulatory cytokines
Abstract
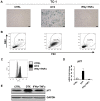
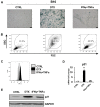
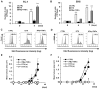
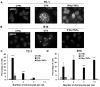
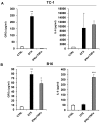
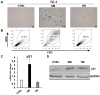
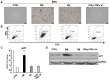
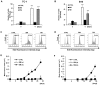
Cellular senescence is the process of the permanent proliferative arrest of cells in response to various inducers. It is accompanied by typical morphological changes, in addition to the secretion of bioactive molecules, including proinflammatory cytokines and chemokines [known as the senescence-associated secretory phenotype (SASP)]. Thus, senescent cells may affect their local environment and induce a so-called 'bystander' senescence through the state of SASP. The phenotypes of senescent cells are determined by the type of agent inducing cellular stress and the cell lineages. To characterise the phenotypes of senescent cancer cells, two murine cell lines were employed in the present study: TC-1 and B16F10 (B16) cells. Two distinct senescence inductors were used: Chemotherapeutic agent docetaxel (DTX) and a combination of immunomodulatory cytokines, including interferon γ (IFNγ) and tumour necrosis factor α (TNFα). It was demonstrated that DTX induced senescence in TC-1 and B16 tumour cell lines, which was demonstrated by growth arrest, positive β-galactosidase staining, increased p21Waf1 (p21) expression and the typical SASP capable of inducing a 'bystander' senescence. By contrast, treatment with a combination of T helper cell 1 cytokines, IFNγ and TNFα, induced proliferation arrest only in B16 cells. Despite the presence of certain characteristic features resembling senescent cells (proliferation arrest, morphological changes and increased p21 expression), these cells were able to form tumours in vivo and started to proliferate upon cytokine withdrawal. In addition, B16 cells were not able to induce a 'bystander' senescence. In summary, the present study described cell line- and treatment-associated differences in the phenotypes of senescent cells that may be relevant in optimization of cancer chemo- and immunotherapy.
Figures
Similar articles
-
Senescence-associated secretory factors induced by cisplatin in melanoma cells promote non-senescent melanoma cell growth through activation of the ERK1/2-RSK1 pathway.Cell Death Dis. 2018 Feb 15;9(3):260. doi: 10.1038/s41419-018-0303-9. Cell Death Dis. 2018. PMID: 29449532 Free PMC article.
-
Tumor growth accelerated by chemotherapy-induced senescent cells is suppressed by treatment with IL-12 producing cellular vaccines.Oncotarget. 2016 Aug 23;7(34):54952-54964. doi: 10.18632/oncotarget.10712. Oncotarget. 2016. PMID: 27448982 Free PMC article.
-
IFN-γ and TNF Induce Senescence and a Distinct Senescence-Associated Secretory Phenotype in Melanoma.Cells. 2022 Apr 30;11(9):1514. doi: 10.3390/cells11091514. Cells. 2022. PMID: 35563820 Free PMC article.
-
Cellular senescence in the development and treatment of cancer.Curr Pharm Des. 2010 Jan;16(1):79-100. doi: 10.2174/138161210789941874. Curr Pharm Des. 2010. PMID: 20214620 Review.
-
Cellular senescence in ageing, age-related disease and longevity.Curr Vasc Pharmacol. 2014;12(5):698-706. doi: 10.2174/1570161111666131219094045. Curr Vasc Pharmacol. 2014. PMID: 24350932 Review.
Cited by
-
Polyploid giant cancer cells: origin, possible pathways of formation, characteristics, and mechanisms of regulation.Front Cell Dev Biol. 2024 Jul 11;12:1410637. doi: 10.3389/fcell.2024.1410637. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39055650 Free PMC article. Review.
-
Enhanced target-specific delivery of docetaxel-loaded nanoparticles using engineered T cell receptors.Nanoscale. 2021 Sep 17;13(35):15010-15020. doi: 10.1039/d1nr04001d. Nanoscale. 2021. PMID: 34533174 Free PMC article.
-
Senescent Secretome of Blind Mole Rat Spalax Inhibits Malignant Behavior of Human Breast Cancer Cells Triggering Bystander Senescence and Targeting Inflammatory Response.Int J Mol Sci. 2023 Mar 7;24(6):5132. doi: 10.3390/ijms24065132. Int J Mol Sci. 2023. PMID: 36982207 Free PMC article.
-
Sudocetaxel Zendusortide (TH1902) triggers the cGAS/STING pathway and potentiates anti-PD-L1 immune-mediated tumor cell killing.Front Immunol. 2024 Feb 16;15:1355945. doi: 10.3389/fimmu.2024.1355945. eCollection 2024. Front Immunol. 2024. PMID: 38482021 Free PMC article.
-
Senescent Tumor Cells in the Peritoneal Carcinomatosis Drive Immunosenescence in the Tumor Microenvironment.Front Immunol. 2022 Jun 30;13:908449. doi: 10.3389/fimmu.2022.908449. eCollection 2022. Front Immunol. 2022. PMID: 35844581 Free PMC article.
References
-
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-asso ciated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

