Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs
- PMID: 30224312
- PMCID: PMC6197674
- DOI: 10.1016/j.ebiom.2018.09.005
Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs
Abstract
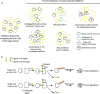
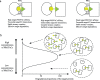
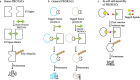
There are several challenges towards the development and clinical use of small molecule inhibitors, which are currently the main type of targeted therapies towards intracellular proteins. PROteolysis-TArgeting Chimeras (PROTACs) exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins. Recently, small-molecule PROTACs with high potency have been frequently reported. In this review, we summarize the emerging characteristics of small-molecule PROTACs, such as inducing a rapid, profound and sustained degradation, inducing a robust inhibition of downstream signals, displaying enhanced target selectivity, and overcoming resistance to small molecule inhibitors. In tumor xenografts, small-molecule PROTACs can significantly attenuate tumor progression. In addition, we also introduce recent developments of the PROTAC technology such as homo-PROTACs. The outstanding advantages over traditional small-molecule drugs and the promising preclinical data suggest that small-molecule PROTAC technology has the potential to greatly promote the development of targeted therapy drugs.
Keywords: E3 ligases; Induced protein degradation; PROTAC; Targeted therapy drugs; Ubiquitin-proteasome system.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Small-molecule PROTACs: novel agents for cancer therapy.Future Med Chem. 2020 May;12(10):915-938. doi: 10.4155/fmc-2019-0340. Epub 2020 Apr 9. Future Med Chem. 2020. PMID: 32270707 Review.
-
Small molecule PROTACs in targeted therapy: An emerging strategy to induce protein degradation.Eur J Med Chem. 2019 Jul 15;174:159-180. doi: 10.1016/j.ejmech.2019.04.036. Epub 2019 Apr 19. Eur J Med Chem. 2019. PMID: 31035238 Review.
-
PROTAC-DB: an online database of PROTACs.Nucleic Acids Res. 2021 Jan 8;49(D1):D1381-D1387. doi: 10.1093/nar/gkaa807. Nucleic Acids Res. 2021. PMID: 33010159 Free PMC article.
-
Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead.Cell Chem Biol. 2018 Jan 18;25(1):78-87.e5. doi: 10.1016/j.chembiol.2017.09.010. Epub 2017 Nov 9. Cell Chem Biol. 2018. PMID: 29129718 Free PMC article.
-
[Induced degradation of proteins by PROTACs and other strategies: towards promising drugs].Biol Aujourdhui. 2021;215(1-2):25-43. doi: 10.1051/jbio/2021007. Epub 2021 Aug 16. Biol Aujourdhui. 2021. PMID: 34397373 French.
Cited by
-
Tackling Drug Resistance in EGFR Exon 20 Insertion Mutant Lung Cancer.Pharmgenomics Pers Med. 2021 Mar 9;14:301-317. doi: 10.2147/PGPM.S242045. eCollection 2021. Pharmgenomics Pers Med. 2021. PMID: 33727854 Free PMC article. Review.
-
A biphenyl inhibitor of eIF4E targeting an internal binding site enables the design of cell-permeable PROTAC-degraders.Eur J Med Chem. 2021 Jul 5;219:113435. doi: 10.1016/j.ejmech.2021.113435. Epub 2021 Apr 8. Eur J Med Chem. 2021. PMID: 33892272 Free PMC article.
-
Multiple Myeloma Therapy: Emerging Trends and Challenges.Cancers (Basel). 2022 Aug 23;14(17):4082. doi: 10.3390/cancers14174082. Cancers (Basel). 2022. PMID: 36077618 Free PMC article. Review.
-
WWP1 E3 ligase at the crossroads of health and disease.Cell Death Dis. 2023 Dec 21;14(12):853. doi: 10.1038/s41419-023-06380-0. Cell Death Dis. 2023. PMID: 38129384 Free PMC article. Review.
-
Discovery of an AKT Degrader with Prolonged Inhibition of Downstream Signaling.Cell Chem Biol. 2020 Jan 16;27(1):66-73.e7. doi: 10.1016/j.chembiol.2019.11.014. Epub 2019 Dec 16. Cell Chem Biol. 2020. PMID: 31859249 Free PMC article.
References
-
- Lavanya V., Mohamed Adil A.A., Neesar A., Arun K.R., Shazia J. Small molecule inhibitors as emerging cancer therapeutics. Integr Cancer Sci Ther. 2014;1
-
- Wu P., Nielsen T.E., Clausen M.H. Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discov Today. 2016;21:5–10. - PubMed
-
- Toure M., Crews C.M. Small-molecule PROTACS: new approaches to protein degradation. Angew Chem Int Ed Engl. 2016;55:1966–1973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

