Expression of STING Is Increased in Liver Tissues From Patients With NAFLD and Promotes Macrophage-Mediated Hepatic Inflammation and Fibrosis in Mice
- PMID: 30213555
- PMCID: PMC6279491
- DOI: 10.1053/j.gastro.2018.09.010
Expression of STING Is Increased in Liver Tissues From Patients With NAFLD and Promotes Macrophage-Mediated Hepatic Inflammation and Fibrosis in Mice
Abstract
Background & aims: Transmembrane protein 173 (TMEM173 or STING) signaling by macrophage activates the type I interferon-mediated innate immune response. The innate immune response contributes to hepatic steatosis and non-alcoholic fatty liver disease (NAFLD). We investigated whether STING regulates diet-induced in hepatic steatosis, inflammation, and liver fibrosis in mice.
Methods: Mice with disruption of Tmem173 (STINGgt) on a C57BL/6J background, mice without disruption of this gene (controls), and mice with disruption of Tmem173 only in myeloid cells were fed a standard chow diet, a high-fat diet (HFD; 60% fat calories), or a methionine- and choline-deficient diet (MCD). Liver tissues were collected and analyzed by histology and immunohistochemistry. Bone marrow cells were isolated from mice, differentiated into macrophages, and incubated with 5,6-dimethylxanthenone-4-acetic acid (DMXAA; an activator of STING) or cyclic guanosine monophosphate-adenosine monophosphate (cGAMP). Macrophages or their media were applied to mouse hepatocytes or human hepatic stellate cells (LX2) cells, which were analyzed for cytokine expression, protein phosphorylation, and fat deposition (by oil red O staining after incubation with palmitate). We obtained liver tissues from patients with and without NAFLD and analyzed these by immunohistochemistry.
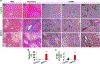
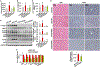
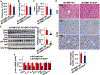
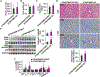
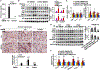
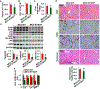
Results: Non-parenchymal cells of liver tissues from patients with NAFLD had higher levels of STING than cells of liver tissues from patients without NAFLD. STINGgt mice and mice with disruption only in myeloid cells developed less severe hepatic steatosis, inflammation, and/or fibrosis after the HFD or MCD than control mice. Levels of phosphorylated c-Jun N-terminal kinase and p65 and mRNAs encoding tumor necrosis factor and interleukins 1B and 6 (markers of inflammation) were significantly lower in liver tissues from STINGgt mice vs control mice after the HFD or MCD. Transplantation of bone marrow cells from control mice to STINGgt mice restored the severity of steatosis and inflammation after the HFD. Macrophages from control, but not STINGgt, mice increased markers of inflammation in response to lipopolysaccharide and cGAMP. Hepatocytes and stellate cells cocultured with STINGgt macrophages in the presence of DMXAA or incubated with the medium collected from these macrophages had decreased fat deposition and markers of inflammation compared with hepatocytes or stellate cells incubated with control macrophages.
Conclusions: Levels of STING were increased in liver tissues from patients with NAFLD and mice with HFD-induced steatosis. In mice, loss of STING from macrophages decreased the severity of liver fibrosis and the inflammatory response. STING might be a therapeutic target for NAFLD.
Keywords: Hepatic Stellate Cell; Interferon; Lipopolysaccharide; Nonalcoholic Steatohepatitis.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
This material, in part, is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas. The content is the responsibility of the author(s) alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the United States Government.
The authors have nothing to declare.
Figures

Comment in
-
Macrophages Steal STING From the Infectious Disease Playbook to Promote Nonalcoholic Fatty Liver Disease.Gastroenterology. 2018 Dec;155(6):1687-1688. doi: 10.1053/j.gastro.2018.11.009. Epub 2018 Nov 9. Gastroenterology. 2018. PMID: 30419212 Free PMC article. No abstract available.
Similar articles
-
Expression of Vsig4 attenuates macrophage-mediated hepatic inflammation and fibrosis in high fat diet (HFD)-induced mice.Biochem Biophys Res Commun. 2019 Aug 27;516(3):858-865. doi: 10.1016/j.bbrc.2019.06.045. Epub 2019 Jun 29. Biochem Biophys Res Commun. 2019. PMID: 31266632
-
Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Nonalcoholic Steatohepatitis.Gastroenterology. 2019 Sep;157(3):777-792.e14. doi: 10.1053/j.gastro.2019.05.002. Epub 2019 May 9. Gastroenterology. 2019. PMID: 31078624
-
STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis.J Clin Invest. 2019 Feb 1;129(2):546-555. doi: 10.1172/JCI121842. Epub 2018 Dec 18. J Clin Invest. 2019. PMID: 30561388 Free PMC article.
-
Decoding cell death signals in liver inflammation.J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
-
Rescue of Hepatic Phospholipid Remodeling Defectin iPLA2β-Null Mice Attenuates Obese but Not Non-Obese Fatty Liver.Biomolecules. 2020 Sep 17;10(9):1332. doi: 10.3390/biom10091332. Biomolecules. 2020. PMID: 32957701 Free PMC article. Review.
Cited by
-
The effects of cGAS-STING inhibition in liver disease, kidney disease, and cellular senescence.Front Immunol. 2024 Jul 24;15:1346446. doi: 10.3389/fimmu.2024.1346446. eCollection 2024. Front Immunol. 2024. PMID: 39114669 Free PMC article. Review.
-
Advancements in tyrosine kinase-mediated regulation of innate nucleic acid sensing.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jan 19;53(1):35-46. doi: 10.3724/zdxbyxb-2023-0480. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38426691 Free PMC article. Review. Chinese, English.
-
Immune and Metabolic Alterations in Liver Fibrosis: A Disruption of Oxygen Homeostasis?Front Mol Biosci. 2022 Feb 3;8:802251. doi: 10.3389/fmolb.2021.802251. eCollection 2021. Front Mol Biosci. 2022. PMID: 35187072 Free PMC article. Review.
-
Current understanding of the cGAS-STING signaling pathway: Structure, regulatory mechanisms, and related diseases.Zool Res. 2023 Jan 18;44(1):183-218. doi: 10.24272/j.issn.2095-8137.2022.464. Zool Res. 2023. PMID: 36579404 Free PMC article. Review.
-
Dihydromyricetin-Encapsulated Liposomes Inhibit Exhaustive Exercise-Induced Liver Inflammation by Orchestrating M1/M2 Macrophage Polarization.Front Pharmacol. 2022 Jun 2;13:887263. doi: 10.3389/fphar.2022.887263. eCollection 2022. Front Pharmacol. 2022. PMID: 35721117 Free PMC article.
References
-
- Sanyal AJ. Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol 2005;2:46–53. - PubMed
-
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. - PubMed
-
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006;43:S99–S112. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK108959/DK/NIDDK NIH HHS/United States
- R01 DK095862/DK/NIDDK NIH HHS/United States
- R21 AA025157/AA/NIAAA NIH HHS/United States
- R21 AA025997/AA/NIAAA NIH HHS/United States
- R01 DK062975/DK/NIDDK NIH HHS/United States
- R01 HL134934/HL/NHLBI NIH HHS/United States
- R01 DK110035/DK/NIDDK NIH HHS/United States
- R01 DK054811/DK/NIDDK NIH HHS/United States
- R01 DK107310/DK/NIDDK NIH HHS/United States
- I01 BX000574/BX/BLRD VA/United States
- I01 BX001724/BX/BLRD VA/United States
- R01 DK076898/DK/NIDDK NIH HHS/United States
- I01 BX003031/BX/BLRD VA/United States
- R01 DK115184/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

