Targeting VGLUT2 in Mature Dopamine Neurons Decreases Mesoaccumbal Glutamatergic Transmission and Identifies a Role for Glutamate Co-release in Synaptic Plasticity by Increasing Baseline AMPA/NMDA Ratio
- PMID: 30210305
- PMCID: PMC6123381
- DOI: 10.3389/fncir.2018.00064
Targeting VGLUT2 in Mature Dopamine Neurons Decreases Mesoaccumbal Glutamatergic Transmission and Identifies a Role for Glutamate Co-release in Synaptic Plasticity by Increasing Baseline AMPA/NMDA Ratio
Abstract
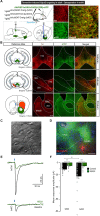
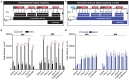
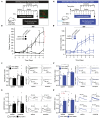
Expression of the Vglut2/Slc17a6 gene encoding the Vesicular glutamate transporter 2 (VGLUT2) in midbrain dopamine (DA) neurons enables these neurons to co-release glutamate in the nucleus accumbens (NAc), a feature of putative importance to drug addiction. For example, it has been shown that conditional deletion of Vglut2 gene expression within developing DA neurons in mice causes altered locomotor sensitization to addictive drugs, such as amphetamine and cocaine, in adulthood. Alterations in DA neurotransmission in the mesoaccumbal pathway has been proposed to contribute to these behavioral alterations but the underlying molecular mechanism remains largely elusive. Repeated exposure to cocaine is known to cause lasting adaptations of excitatory synaptic transmission onto medium spiny neurons (MSNs) in the NAc, but the putative contribution of VGLUT2-mediated glutamate co-release from the mesoaccumbal projection has never been investigated. In this study, we implemented a tamoxifen-inducible Cre-LoxP strategy to selectively probe VGLUT2 in mature DA neurons of adult mice. Optogenetics-coupled patch clamp analysis in the NAc demonstrated a significant reduction of glutamatergic neurotransmission, whilst behavioral analysis revealed a normal locomotor sensitization to amphetamine and cocaine. When investigating if the reduced level of glutamate co-release from DA neurons caused a detectable post-synaptic effect on MSNs, patch clamp analysis identified an enhanced baseline AMPA/NMDA ratio in DA receptor subtype 1 (DRD1)-expressing accumbal MSNs which occluded the effect of cocaine on synaptic transmission. We conclude that VGLUT2 in mature DA neurons actively contributes to glutamatergic neurotransmission in the NAc, a finding which for the first time highlights VGLUT2-mediated glutamate co-release in the complex mechanisms of synaptic plasticity in drug addiction.
Keywords: addiction; amphetamine; cocaine; medium spiny neurons; striatum; substance use disorder; ventral tegmental area (VTA).
Figures
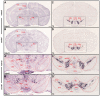
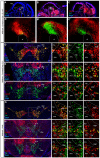
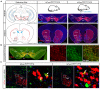
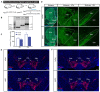
Similar articles
-
Amphetamine-induced plasticity of AMPA receptors in the ventral tegmental area: effects on extracellular levels of dopamine and glutamate in freely moving rats.J Neurosci. 2001 Aug 15;21(16):6362-9. doi: 10.1523/JNEUROSCI.21-16-06362.2001. J Neurosci. 2001. PMID: 11487659 Free PMC article.
-
Deletion of VGLUT2 in midbrain dopamine neurons attenuates dopamine and glutamate responses to methamphetamine in mice.Pharmacol Biochem Behav. 2021 Mar;202:173104. doi: 10.1016/j.pbb.2021.173104. Epub 2021 Jan 12. Pharmacol Biochem Behav. 2021. PMID: 33444596 Free PMC article.
-
Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area.Science. 2013 Sep 27;341(6153):1521-5. doi: 10.1126/science.1237059. Science. 2013. PMID: 24072923
-
Dopamine-glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching.Neurochem Int. 2019 Oct;129:104482. doi: 10.1016/j.neuint.2019.104482. Epub 2019 Jun 3. Neurochem Int. 2019. PMID: 31170424 Free PMC article. Review.
-
Differential striatal spine pathology in Parkinson's disease and cocaine addiction: a key role of dopamine?Neuroscience. 2013 Oct 22;251:2-20. doi: 10.1016/j.neuroscience.2013.07.011. Epub 2013 Jul 16. Neuroscience. 2013. PMID: 23867772 Free PMC article. Review.
Cited by
-
Sex-dependent interactions between prodromal intestinal inflammation and LRRK2 G2019S in mice promote endophenotypes of Parkinson's disease.Commun Biol. 2024 May 15;7(1):570. doi: 10.1038/s42003-024-06256-9. Commun Biol. 2024. PMID: 38750146 Free PMC article.
-
Glutamate Cotransmission in Cholinergic, GABAergic and Monoamine Systems: Contrasts and Commonalities.Front Neural Circuits. 2018 Dec 18;12:113. doi: 10.3389/fncir.2018.00113. eCollection 2018. Front Neural Circuits. 2018. PMID: 30618649 Free PMC article. Review.
-
Audiogenic Seizures in the Fmr1 Knock-Out Mouse Are Induced by Fmr1 Deletion in Subcortical, VGlut2-Expressing Excitatory Neurons and Require Deletion in the Inferior Colliculus.J Neurosci. 2019 Dec 4;39(49):9852-9863. doi: 10.1523/JNEUROSCI.0886-19.2019. Epub 2019 Oct 30. J Neurosci. 2019. PMID: 31666356 Free PMC article.
-
Spatial Distribution of Neurons Expressing Single, Double, and Triple Molecular Characteristics of Glutamatergic, Dopaminergic, or GABAergic Neurons in the Mouse Ventral Tegmental Area.J Mol Neurosci. 2023 Jun;73(6):345-362. doi: 10.1007/s12031-023-02121-2. Epub 2023 May 27. J Mol Neurosci. 2023. PMID: 37243808
-
Dysfunction of ventral tegmental area GABA neurons causes mania-like behavior.Mol Psychiatry. 2021 Sep;26(9):5213-5228. doi: 10.1038/s41380-020-0810-9. Epub 2020 Jun 17. Mol Psychiatry. 2021. PMID: 32555422 Free PMC article.
References
-
- Alsiö J., Nordenankar K., Arvidsson E., Birgner C., Mahmoudi S., Halbout B., et al. . (2011). Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J. Neurosci. 31, 12593–12603. 10.1523/JNEUROSCI.2397-11.2011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

