Dual PAK4-NAMPT Inhibition Impacts Growth and Survival, and Increases Sensitivity to DNA-Damaging Agents in Waldenström Macroglobulinemia
- PMID: 30206161
- PMCID: PMC6320280
- DOI: 10.1158/1078-0432.CCR-18-1776
Dual PAK4-NAMPT Inhibition Impacts Growth and Survival, and Increases Sensitivity to DNA-Damaging Agents in Waldenström Macroglobulinemia
Abstract
Purpose: p21-activated kinase 4 (PAK4) plays a significant biological and functional role in a number of malignancies, including multiple myeloma (MM). On the basis of our promising findings in MM, we here characterize PAK4 expression and role in WM cells, as well effect of dual PAK4-NAMPT inhibitor (KPT-9274) against WM cell growth and viability.
Experimental design: We have analyzed mRNA and protein expression levels of PAK4 in WM cells, and used loss-of-function approach to investigate its contribution to WM cell viability. We have further tested the in vitro and in vivo effect of KPT-9274 against WM cell growth and viability.
Results: We report here high-level expression and functional role of PAK4 in WM, as demonstrated by shRNA-mediated knockdown; and significant impact of KPT-9274 on WM cell growth and viability. The growth inhibitory effect of KPT-9274 was associated with decreased PAK4 expression and NAMPT activity, as well as induction of apoptosis. Interestingly, in WM cell lines treated with KPT-9274, we detected a significant impact on DNA damage and repair genes. Moreover, we observed that apart from inducing DNA damage, KPT-9274 specifically decreased RAD51 and the double-strand break repair by the homologous recombination pathway. As a result, when combined with a DNA alkylating agents bendamustine and melphalan, KPT-9274 provided a synergistic inhibition of cell viability in WM cell lines and primary patient WM cells in vitro and in vivo.
Conclusions: These results support the clinical investigation of KPT-9274 in combination with DNA-damaging agent for treatment of WM.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures
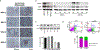

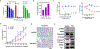
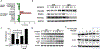


Similar articles
-
Dual and Specific Inhibition of NAMPT and PAK4 By KPT-9274 Decreases Kidney Cancer Growth.Mol Cancer Ther. 2016 Sep;15(9):2119-29. doi: 10.1158/1535-7163.MCT-16-0197. Epub 2016 Jul 7. Mol Cancer Ther. 2016. PMID: 27390344 Free PMC article.
-
KPT-9274, an Inhibitor of PAK4 and NAMPT, Leads to Downregulation of mTORC2 in Triple Negative Breast Cancer Cells.Chem Res Toxicol. 2020 Feb 17;33(2):482-491. doi: 10.1021/acs.chemrestox.9b00376. Epub 2020 Jan 9. Chem Res Toxicol. 2020. PMID: 31876149 Free PMC article.
-
PAK4-NAMPT Dual Inhibition Sensitizes Pancreatic Neuroendocrine Tumors to Everolimus.Mol Cancer Ther. 2021 Oct;20(10):1836-1845. doi: 10.1158/1535-7163.MCT-20-1105. Epub 2021 Jul 12. Mol Cancer Ther. 2021. PMID: 34253597 Free PMC article.
-
Targeting the vulnerability to NAD+ depletion in B-cell acute lymphoblastic leukemia.Leukemia. 2018 Mar;32(3):616-625. doi: 10.1038/leu.2017.281. Epub 2017 Sep 14. Leukemia. 2018. PMID: 28904384
-
Recent advances on development of p21-activated kinase 4 inhibitors as anti-tumor agents.Front Pharmacol. 2022 Aug 29;13:956220. doi: 10.3389/fphar.2022.956220. eCollection 2022. Front Pharmacol. 2022. PMID: 36105226 Free PMC article. Review.
Cited by
-
enAsCas12a Enables CRISPR-Directed Evolution to Screen for Functional Drug Resistance Mutations in Sequences Inaccessible to SpCas9.Mol Ther. 2021 Jan 6;29(1):208-224. doi: 10.1016/j.ymthe.2020.09.025. Epub 2020 Sep 20. Mol Ther. 2021. PMID: 33002419 Free PMC article.
-
Remodeling of the tumor microenvironment through PAK4 inhibition sensitizes tumors to immune checkpoint blockade.Cancer Res Commun. 2022 Oct;2(10):1214-1228. doi: 10.1158/2767-9764.crc-21-0133. Epub 2022 Oct 19. Cancer Res Commun. 2022. PMID: 36588582 Free PMC article.
-
CORO1C, a novel PAK4 binding protein, recruits phospho-PAK4 at serine 99 to the leading edge and promotes the migration of gastric cancer cells.Acta Biochim Biophys Sin (Shanghai). 2022 May 25;54(5):673-685. doi: 10.3724/abbs.2022044. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35593474 Free PMC article.
-
Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials.Sci Transl Med. 2019 Sep 11;11(509):eaaw8412. doi: 10.1126/scitranslmed.aaw8412. Sci Transl Med. 2019. PMID: 31511426 Free PMC article.
-
PAK4 and NAMPT as Novel Therapeutic Targets in Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, and Mantle Cell Lymphoma.Cancers (Basel). 2021 Dec 29;14(1):160. doi: 10.3390/cancers14010160. Cancers (Basel). 2021. PMID: 35008323 Free PMC article.
References
-
- Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014;123(11):1637–46 doi 10.1182/blood-2013-09-525808. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

