RAS-expanded Mutations and HER2 Expression in Metastatic Colorectal Cancer: A New Step of Precision Medicine
- PMID: 30199395
- PMCID: PMC6135466
- DOI: 10.1097/PAI.0000000000000475
RAS-expanded Mutations and HER2 Expression in Metastatic Colorectal Cancer: A New Step of Precision Medicine
Abstract
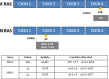
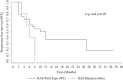
Cetuximab and panitumumab monoclonal antibodies are a milestone in the history of treatment of metastatic colorectal cancer (mCRC) and point toward future directions for personalized treatment. Recent studies have shown that broader RAS testing is needed to select patients for targeted therapy. The objectives of our study were to identify the prevalence of RAS mutations and evaluate human epidermal growth factor receptor 2 (HER2) expression in KRAS exon 2 wild-type (WT) mCRC patients, correlating the findings with objective response rate, progression-free survival, and overall survival. In total, 29 mCRC patients undergoing treatment with cetuximab therapy were enrolled in this study. By pyrosequencing, mutations were found in 17% of nonresponder patients, in KRAS codon 146 and NRAS codon 12. HER2 positivity was limited to only 1 responder carcinoma specimen. There was no correlation between RAS mutation, HER2/neu expression, and clinicopathologic findings. We highlighted significantly the differences between objective response rate and RAS gene status. The overall survival and progression-free survival of RAS WT patients were higher compared with those with RAS-mutated disease. Clinical response to cetuximab therapy is impaired in the presence of RAS-expanded mutations. In fact, our finding of 5 mutations in RAS-expanded genes allowed us to understand the resistance to cetuximab in 33% of KRAS WT exon 2 nonresponder patients. HER2 does not seem to be a potential biomarker for cetuximab-targeted therapy. These analyses suggest that the assessment of other biomarkers is needed to determine the best treatment for patients with mCRC, to maximize benefit and minimize harm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer.J Clin Oncol. 2008 Dec 10;26(35):5705-12. doi: 10.1200/JCO.2008.18.0786. Epub 2008 Nov 10. J Clin Oncol. 2008. PMID: 19001320
-
Efficacy and Toxicity of Panitumumab After Progression on Cetuximab and Predictive Value of MiR-31-5p in Metastatic Wild-type KRAS Colorectal Cancer Patients.Anticancer Res. 2016 Sep;36(9):4955-9. doi: 10.21873/anticanres.11063. Anticancer Res. 2016. PMID: 27630355 Clinical Trial.
-
Neratinib-Plus-Cetuximab in Quadruple-WT (KRAS, NRAS, BRAF, PIK3CA) Metastatic Colorectal Cancer Resistant to Cetuximab or Panitumumab: NSABP FC-7, A Phase Ib Study.Clin Cancer Res. 2021 Mar 15;27(6):1612-1622. doi: 10.1158/1078-0432.CCR-20-1831. Epub 2020 Nov 17. Clin Cancer Res. 2021. PMID: 33203645 Free PMC article. Clinical Trial.
-
HER2 as a Predictive Biomarker and Treatment Target in Colorectal Cancer.Clin Colorectal Cancer. 2020 Jun;19(2):65-72. doi: 10.1016/j.clcc.2020.02.007. Epub 2020 Feb 8. Clin Colorectal Cancer. 2020. PMID: 32229076 Review.
-
The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis.Acta Oncol. 2014 Jul;53(7):852-64. doi: 10.3109/0284186X.2014.895036. Epub 2014 Mar 25. Acta Oncol. 2014. PMID: 24666267 Review.
Cited by
-
Different Clinicopathological Characteristics in Indonesian Colorectal Patients with NRAS Mutations and HER2 Over-Expression.Asian Pac J Cancer Prev. 2023 Apr 1;24(4):1373-1377. doi: 10.31557/APJCP.2023.24.4.1373. Asian Pac J Cancer Prev. 2023. PMID: 37116161 Free PMC article.
-
PD-L1 expression in colorectal cancer defines three subsets of tumor immune microenvironments.Oncotarget. 2018 Jan 12;9(9):8584-8596. doi: 10.18632/oncotarget.24196. eCollection 2018 Feb 2. Oncotarget. 2018. PMID: 29492219 Free PMC article.
-
The predictive value of KRAS and NRAS mutations in metastatic colorectal cancer.Med J Islam Repub Iran. 2020 Apr 27;34:39. doi: 10.34171/mjiri.34.39. eCollection 2020. Med J Islam Repub Iran. 2020. PMID: 32617278 Free PMC article. No abstract available.
-
CircTBL1XR1/miR-424 axis regulates Smad7 to promote the proliferation and metastasis of colorectal cancer.J Gastrointest Oncol. 2020 Oct;11(5):918-931. doi: 10.21037/jgo-20-395. J Gastrointest Oncol. 2020. PMID: 33209488 Free PMC article.
-
Prevalence, prognosis and predictive status of HER2 amplification in anti-EGFR-resistant metastatic colorectal cancer.Clin Transl Oncol. 2020 Jun;22(6):813-822. doi: 10.1007/s12094-019-02213-9. Epub 2019 Oct 5. Clin Transl Oncol. 2020. PMID: 31587152 Review.
References
-
- Sorich MJ, Wiese MD, Rowland A, et al. Extended mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26:13–21. - PubMed
-
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. - PubMed
-
- Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. - PubMed
-
- Wong NACS, Gonzales D, Salto-Tellez M, et al. RAS testing of colorectal carcinoma-a guidance document from the Association of Clinical Pathologists Molecular Pathology and Diagnostics Group. J Clin Pathol. 2014;67:751–757. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

