Relationship of Transmural Variations in Myofiber Contractility to Left Ventricular Ejection Fraction: Implications for Modeling Heart Failure Phenotype With Preserved Ejection Fraction
- PMID: 30197595
- PMCID: PMC6117406
- DOI: 10.3389/fphys.2018.01003
Relationship of Transmural Variations in Myofiber Contractility to Left Ventricular Ejection Fraction: Implications for Modeling Heart Failure Phenotype With Preserved Ejection Fraction
Abstract
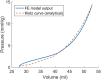
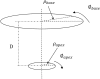
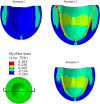
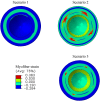
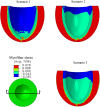
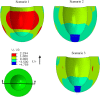
The pathophysiological mechanisms underlying preserved left ventricular (LV) ejection fraction (EF) in patients with heart failure and preserved ejection fraction (HFpEF) remain incompletely understood. We hypothesized that transmural variations in myofiber contractility with existence of subendocardial dysfunction and compensatory increased subepicardial contractility may underlie preservation of LVEF in patients with HFpEF. We quantified alterations in myocardial function in a mathematical model of the human LV that is based on the finite element method. The fiber-reinforced material formulation of the myocardium included passive and active properties. The passive material properties were determined such that the diastolic pressure-volume behavior of the LV was similar to that shown in published clinical studies of pressure-volume curves. To examine changes in active properties, we considered six scenarios: (1) normal properties throughout the LV wall; (2) decreased myocardial contractility in the subendocardium; (3) increased myocardial contractility in the subepicardium; (4) myocardial contractility decreased equally in all layers, (5) myocardial contractility decreased in the midmyocardium and subepicardium, (6) myocardial contractility decreased in the subepicardium. Our results indicate that decreased subendocardial contractility reduced LVEF from 53.2 to 40.5%. Increased contractility in the subepicardium recovered LVEF from 40.5 to 53.2%. Decreased contractility transmurally reduced LVEF and could not be recovered if subepicardial and midmyocardial contractility remained depressed. The computational results simulating the effects of transmural alterations in the ventricular tissue replicate the phenotypic patterns of LV dysfunction observed in clinical practice. In particular, data for LVEF, strain and displacement are consistent with previous clinical observations in patients with HFpEF, and substantiate the hypothesis that increased subepicardial contractility may compensate for subendocardial dysfunction and play a vital role in maintaining LVEF.
Keywords: finite element method; heart failure and preserved ejection fraction; left ventricle; myocardial contractility; simulation.
Figures



Similar articles
-
A transmural gradient of myocardial remodeling in early-stage heart failure with preserved ejection fraction in the pig.J Anat. 2020 Mar;236(3):531-539. doi: 10.1111/joa.13117. Epub 2019 Nov 21. J Anat. 2020. PMID: 31749243 Free PMC article.
-
Computational Modeling Studies of the Roles of Left Ventricular Geometry, Afterload, and Muscle Contractility on Myocardial Strains in Heart Failure with Preserved Ejection Fraction.J Cardiovasc Transl Res. 2021 Dec;14(6):1131-1145. doi: 10.1007/s12265-021-10130-y. Epub 2021 Apr 29. J Cardiovasc Transl Res. 2021. PMID: 33928526 Free PMC article.
-
Myocardial contractile dysfunction associated with increased 3-month and 1-year mortality in hospitalized patients with heart failure and preserved ejection fraction.Int J Cardiol. 2013 Oct 3;168(3):1975-83. doi: 10.1016/j.ijcard.2012.12.084. Epub 2013 Jan 19. Int J Cardiol. 2013. PMID: 23336957
-
[Transmural heterogeneity of the left ventricular wall: subendocardial layer and subepicardial layer].J Cardiol. 2000 Mar;35(3):205-18. J Cardiol. 2000. PMID: 10808428 Review. Japanese.
-
A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation.J Am Coll Cardiol. 2013 Jul 23;62(4):263-71. doi: 10.1016/j.jacc.2013.02.092. Epub 2013 May 15. J Am Coll Cardiol. 2013. PMID: 23684677 Review.
Cited by
-
Intramyocardial Injections to De-Stiffen the Heart: A Subject-Specific in Silico Approach.Mol Cell Biomech. 2019;16(3):185-197. doi: 10.32604/mcb.2019.07364. Mol Cell Biomech. 2019. PMID: 32063808 Free PMC article.
-
Establishing the Biofidelity of a Multiphysics Finite Element Model of the Human Heart.Cardiovasc Eng Technol. 2021 Aug;12(4):387-397. doi: 10.1007/s13239-021-00538-7. Epub 2021 Apr 13. Cardiovasc Eng Technol. 2021. PMID: 33851325
-
Prediction of Left Ventricular Mechanics Using Machine Learning.Front Phys. 2019 Sep;7:117. doi: 10.3389/fphy.2019.00117. Epub 2019 Sep 6. Front Phys. 2019. PMID: 31903394 Free PMC article.
-
Patient-Specific Analysis of Ascending Thoracic Aortic Aneurysm with the Living Heart Human Model.Bioengineering (Basel). 2021 Nov 4;8(11):175. doi: 10.3390/bioengineering8110175. Bioengineering (Basel). 2021. PMID: 34821741 Free PMC article.
-
Application of feed forward and recurrent neural networks in simulation of left ventricular mechanics.Sci Rep. 2020 Dec 18;10(1):22298. doi: 10.1038/s41598-020-79191-4. Sci Rep. 2020. PMID: 33339836 Free PMC article.
References
-
- Adeniran I., MacIver D. H., Hancox J. C., Zhang H. (2015). Abnormal calcium homeostasis in heart failure with preserved ejection fraction is related to both reduced contractile function and incomplete relaxation: an electromechanically detailed biophysical modeling study. Front. Physiol. 6:78. 10.3389/fphys.2015.00078 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

