Preventing Clinically Important Deterioration of COPD with Addition of Umeclidinium to Inhaled Corticosteroid/Long-Acting β2-Agonist Therapy: An Integrated Post Hoc Analysis
- PMID: 30191464
- PMCID: PMC6182634
- DOI: 10.1007/s12325-018-0771-4
Preventing Clinically Important Deterioration of COPD with Addition of Umeclidinium to Inhaled Corticosteroid/Long-Acting β2-Agonist Therapy: An Integrated Post Hoc Analysis
Abstract
Introduction: Assessing clinically important measures of disease progression is essential for evaluating therapeutic effects on disease stability in chronic obstructive pulmonary disease (COPD). This analysis assessed whether providing additional bronchodilation with the long-acting muscarinic antagonist umeclidinium (UMEC) to patients treated with inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA) therapy would improve disease stability compared with ICS/LABA therapy alone.
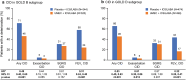
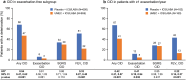
Methods: This integrated post hoc analysis of four 12-week, randomized, double-blind trials (NCT01772134, NCT01772147, NCT01957163, NCT02119286) compared UMEC 62.5 µg with placebo added to open-label ICS/LABA in symptomatic patients with COPD (modified Medical Research Council dyspnea scale score ≥ 2). A clinically important deterioration (CID) was defined as: a decrease from baseline of ≥ 100 mL in trough forced expiratory volume in 1 s (FEV1), an increase from baseline of ≥ 4 units in St George's Respiratory Questionnaire (SGRQ) total score, or a moderate/severe exacerbation. Risk of a first CID was evaluated in the intent-to-treat (ITT) population and in patients stratified by Global initiative for chronic Obstructive Lung Disease (GOLD) classification, exacerbation history and type of ICS/LABA therapy. Adverse events (AEs) were also assessed.
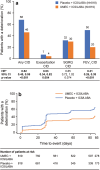
Results: Overall, 1637 patients included in the ITT population received UMEC + ICS/LABA (n = 819) or placebo + ICS/LABA (n = 818). Additional bronchodilation with UMEC reduced the risk of a first CID by 45-58% in the ITT population and all subgroups analyzed compared with placebo (all p < 0.001). Improvements were observed in reducing FEV1 (69% risk reduction; p < 0.001) and exacerbation (47% risk reduction; p = 0.004) events in the ITT population. No significant reduction in risk of a SGRQ CID was observed. AE incidence was similar between treatment groups.
Conclusion: Symptomatic patients with COPD receiving ICS/LABA experience frequent deteriorations. Additional bronchodilation with UMEC significantly reduced the risk of CID and provided greater short-term stability versus continued ICS/LABA therapy in these patients.
Funding: GlaxoSmithKline (study number: 202067). Plain language summary available for this article.
Keywords: Add-on LAMA; COPD; Clinically important deteriorations; Fluticasone furoate/vilanterol; Fluticasone propionate/salmeterol; Respiratory; Triple therapy; Umeclidinium.
Conflict of interest statement
Ian P. Naya is an employee of GSK and holds stocks and shares in GSK. David A. Lipson is an employee of GSK and holds stocks and shares in GSK. Chris Compton is an employee of GSK and holds stocks and shares in GSK. Lee Tombs is a contingent worker on assignment at GSK. ELLIPTA and DISKUS are owned by or licensed to the GSK group of companies.
Figures
Similar articles
-
Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: Results of two randomized studies.Respir Med. 2015 Sep;109(9):1155-63. doi: 10.1016/j.rmed.2015.06.006. Epub 2015 Jun 14. Respir Med. 2015. PMID: 26117292 Clinical Trial.
-
Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol.Int J Chron Obstruct Pulmon Dis. 2016 Jun 24;11:1413-24. doi: 10.2147/COPD.S101612. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27445468 Free PMC article.
-
Real-world effectiveness of umeclidinium/vilanterol versus fluticasone propionate/salmeterol as initial maintenance therapy for chronic obstructive pulmonary disease (COPD): a retrospective cohort study.Int J Chron Obstruct Pulmon Dis. 2019 Aug 1;14:1721-1737. doi: 10.2147/COPD.S204649. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31534326 Free PMC article.
-
Fluticasone Furoate/Umeclidinium/Vilanterol (FF/UMEC/VI) Triple Therapy Compared with Other Therapies for the Treatment of COPD: A Network Meta-Analysis.Adv Ther. 2022 Sep;39(9):3957-3978. doi: 10.1007/s12325-022-02231-0. Epub 2022 Jul 17. Adv Ther. 2022. PMID: 35849317 Free PMC article. Review.
-
Once-daily fluticasone furoate/vilanterol 100/25 mcg versus twice daily combination therapies in COPD - mixed treatment comparisons of clinical efficacy.Respir Res. 2015 Feb 15;16(1):25. doi: 10.1186/s12931-015-0184-8. Respir Res. 2015. PMID: 25849223 Free PMC article. Review.
Cited by
-
Long-term cost and utility consequences of short-term clinically important deterioration in patients with chronic obstructive pulmonary disease: results from the TORCH study.Int J Chron Obstruct Pulmon Dis. 2019 May 3;14:939-951. doi: 10.2147/COPD.S188898. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31190781 Free PMC article. Clinical Trial.
-
Clinically Important Deterioration (CID) and Ageing in COPD: A Systematic Review and Meta-Regression Analysis According to PRISMA Statement.Int J Chron Obstruct Pulmon Dis. 2023 Oct 10;18:2225-2243. doi: 10.2147/COPD.S396945. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 37841747 Free PMC article.
-
Comparation of predictive value of CAT and change in CAT in the short term for future exacerbation of chronic obstructive pulmonary disease.Ann Med. 2022 Dec;54(1):875-885. doi: 10.1080/07853890.2022.2055134. Ann Med. 2022. PMID: 35341416 Free PMC article.
-
Diagnosis and Treatment of Early Chronic Obstructive Lung Disease (COPD).J Clin Med. 2020 Oct 26;9(11):3426. doi: 10.3390/jcm9113426. J Clin Med. 2020. PMID: 33114502 Free PMC article. Review.
-
Long-term outcomes following first short-term clinically important deterioration in COPD.Respir Res. 2018 Nov 20;19(1):222. doi: 10.1186/s12931-018-0928-3. Respir Res. 2018. PMID: 30453972 Free PMC article. Clinical Trial.
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Updated 2018. http://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revi.... Accessed Jan 23, 2018.
-
- World Health Organization (WHO). Chronic obstructive pulmonary disease (COPD) fact sheet. Updated November 2017. http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed Nov 16, 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

