Downregulation of microRNA-1 attenuates glucose-induced apoptosis by regulating the liver X receptor α in cardiomyocytes
- PMID: 30186406
- PMCID: PMC6122156
- DOI: 10.3892/etm.2018.6388
Downregulation of microRNA-1 attenuates glucose-induced apoptosis by regulating the liver X receptor α in cardiomyocytes
Abstract
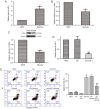
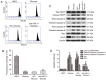
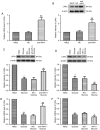
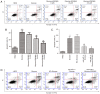
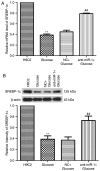
Diabetic cardiomyopathy (DCM) is characterized by abnormal myocardial structure or performance. It has been suggested that microRNA-1 (miR-1) may be abnormally expressed in the hearts of patients with diabetes. In the present study, the role of miR-1 in glucose-induced apoptosis and its underlying mechanism of action was investigated in rat cardiomyocyte H9C2 cells. Cells were transfected with anti-miR-1 or miR-1-overexpression plasmids and the expression of miR-1 and liver X receptor α (LXRα) were determined by reverse transcription-quantitative polymerase chain reaction analysis. The proportion of apoptotic cells was determined using an Annexin-V-FITC apoptosis detection kit and the mitochondrial membrane potential (ΔΨ) was measured following staining with rhodamine 123. In addition, the expression of apoptosis-associated proteins was measured by western blot analysis. The results demonstrated that expression of miR-1 was significantly increased, whereas the expression of LXRα was significantly decreased in H9C2 cells following treatment with glucose. miR-1 knockdown significantly inhibited apoptosis, increased the ΔΨ and suppressed the cleavage of poly (adenosine diphosphate-ribose) polymerase, caspase-3 and caspase-9. It also significantly downregulated the expression of Bcl-2 and upregulated the expression of Bax. In addition, it was demonstrated that miR-1 regulates LXRα; transfection with anti-miR-1 significantly increased the expression of LXRα. Furthermore, treatment of cells with the LXR agonist GW3965 inhibited apoptosis in glucose-induced anti-miR-1 cells. These results suggest a novel function of miR-1: The regulation of cardiomyocyte apoptosis via LXRα, and provide novel insights into regarding the complex mechanisms involved in DCM.
Keywords: apoptosis; cardiomyocytes; diabetic cardiomyopathy; liver X receptor α; microRNA-1.
Figures

Similar articles
-
Liver X receptor α is targeted by microRNA-1 to inhibit cardiomyocyte apoptosis through a ROS-mediated mitochondrial pathway.Biochem Cell Biol. 2018 Feb;96(1):11-18. doi: 10.1139/bcb-2017-0154. Epub 2017 Oct 12. Biochem Cell Biol. 2018. PMID: 29024600
-
MiR-144 affects proliferation and apoptosis of high glucose-induced AC16 cardiomyocytes by regulating CTRP3/JNK signaling.Int J Clin Exp Pathol. 2020 Feb 1;13(2):142-152. eCollection 2020. Int J Clin Exp Pathol. 2020. PMID: 32211094 Free PMC article.
-
Inhibition of long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 attenuates high glucose-induced cardiomyocyte apoptosis via regulation of miR-181a-5p.Exp Anim. 2020 Jan 29;69(1):34-44. doi: 10.1538/expanim.19-0058. Epub 2019 Jul 29. Exp Anim. 2020. PMID: 31353329 Free PMC article.
-
MicroRNA-29b Regulates the Mitochondria-Dependent Apoptotic Pathway by Targeting Bax in Doxorubicin Cardiotoxicity.Cell Physiol Biochem. 2018;48(2):692-704. doi: 10.1159/000491896. Epub 2018 Jul 19. Cell Physiol Biochem. 2018. PMID: 30025410
-
Inhibition of MicroRNA-124 Reduces Cardiomyocyte Apoptosis Following Myocardial Infarction via Targeting STAT3.Cell Physiol Biochem. 2018;51(1):186-200. doi: 10.1159/000495173. Epub 2018 Nov 15. Cell Physiol Biochem. 2018. PMID: 30439699
Cited by
-
The interregulatory circuit between non-coding RNA and apoptotic signaling in diabetic cardiomyopathy.Noncoding RNA Res. 2024 Jun 12;9(4):1080-1097. doi: 10.1016/j.ncrna.2024.06.011. eCollection 2024 Dec. Noncoding RNA Res. 2024. PMID: 39022683 Free PMC article. Review.
-
Cu(II) Coordination Polymer Inhibits Liver Cancer Development via Targeting BCL-2 Protein and Activating Apoptotic Pathway.Dis Markers. 2021 Oct 12;2021:2174290. doi: 10.1155/2021/2174290. eCollection 2021. Dis Markers. 2021. PMID: 35356669 Free PMC article.
-
A Roadmap for Fixing the Heart: RNA Regulatory Networks in Cardiac Disease.Mol Ther Nucleic Acids. 2020 Jun 5;20:673-686. doi: 10.1016/j.omtn.2020.04.007. Epub 2020 Apr 25. Mol Ther Nucleic Acids. 2020. PMID: 32380417 Free PMC article. Review.
-
Identification of miR-1 and miR-499 in chronic atrial fibrillation by bioinformatics analysis and experimental validation.Front Cardiovasc Med. 2024 Aug 16;11:1400643. doi: 10.3389/fcvm.2024.1400643. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39221422 Free PMC article.
-
Connexins and Glucose Metabolism in Cancer.Int J Mol Sci. 2022 Sep 5;23(17):10172. doi: 10.3390/ijms231710172. Int J Mol Sci. 2022. PMID: 36077565 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
