The Curcumin Analogs 2-Pyridyl Cyclohexanone Induce Apoptosis via Inhibition of the JAK2-STAT3 Pathway in Human Esophageal Squamous Cell Carcinoma Cells
- PMID: 30186159
- PMCID: PMC6113578
- DOI: 10.3389/fphar.2018.00820
The Curcumin Analogs 2-Pyridyl Cyclohexanone Induce Apoptosis via Inhibition of the JAK2-STAT3 Pathway in Human Esophageal Squamous Cell Carcinoma Cells
Abstract
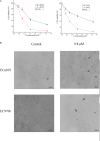
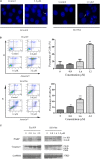
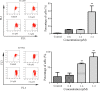
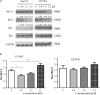
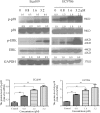
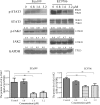
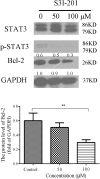
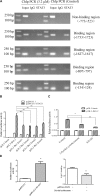
Multiple modifications to the structure of curcumin have been investigated with an aim to improve its potency and biochemical properties. Previously, we have synthesized a series of curcumin analogs. In the present study, the anticancer effect of 2-pyridyl cyclohexanone, one of the curcumin analogs, on esophageal carcinoma Eca109 and EC9706 cell lines and its molecular mechanisms were investigated. 2-Pyridyl cyclohexanone inhibited the proliferation of Eca109 and EC9706 cells by inducing apoptosis as indicated by morphological changes, membrane phospholipid phosphatidylserine ectropion, caspase 3 activation, and cleavage of poly(ADP-ribose) polymerase. Mechanistic studies indicated that 2-pyridyl cyclohexanone disrupted mitochondrial membrane potential, disturbed the balance of the Bcl-2 family proteins, and triggered apoptosis via the mitochondria-mediated intrinsic pathway. In 2-pyridine cyclohexanone-treated cells, the phosphorylation levels of JAK2 and STAT3 were dose-dependently decreased and p38 and p-ERK signals were notably activated in a dose-dependent manner. Moreover, we found that the addition of S3I-201, a STAT3 inhibitor, led to a decreased expression level of Bcl-2 in Eca109 cells. The chromatin immunoprecipitation assay demonstrated that STAT3 bound to the promoter of Bcl-2 in the Eca109 cells. Furthermore, the mutation of four STAT3 binding sites (-1733/-1723, -1627/-1617, -807/-797, and -134/-124) on the promote of Bcl-2 gene alone attenuated the transcriptional activation of STAT3. In addition, down-regulation of STAT3 resulted in less of transcriptional activity of STAT3 on Bcl-2 expression. These data provide a potential molecular mechanism of the apoptotic induction function of 2-pyridyl cyclohexanone, and emphasize its important roles as a therapeutic agent for esophageal squamous carcinoma.
Keywords: 2-pyridyl cyclohexanone; Bcl-2; STAT3; apoptosis; human esophageal squamous cell carcinoma.
Figures
Similar articles
-
JAK2 regulation by licochalcone H inhibits the cell growth and induces apoptosis in oral squamous cell carcinoma.Phytomedicine. 2019 Jan;52:60-69. doi: 10.1016/j.phymed.2018.09.180. Epub 2018 Sep 18. Phytomedicine. 2019. PMID: 30599913
-
Epigallocatechin-3-gallate suppresses cell proliferation and promotes apoptosis in Ec9706 and Eca109 esophageal carcinoma cells.Oncol Lett. 2017 Oct;14(4):4391-4395. doi: 10.3892/ol.2017.6712. Epub 2017 Aug 3. Oncol Lett. 2017. PMID: 28943954 Free PMC article.
-
Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells.Pathol Res Pract. 2017 Oct;213(10):1242-1250. doi: 10.1016/j.prp.2017.09.006. Epub 2017 Sep 12. Pathol Res Pract. 2017. PMID: 28964574
-
Down-regulation of STAT3 induces the apoptosis and G1 cell cycle arrest in esophageal carcinoma ECA109 cells.Cancer Cell Int. 2018 Apr 4;18:53. doi: 10.1186/s12935-018-0549-4. eCollection 2018. Cancer Cell Int. 2018. PMID: 29636641 Free PMC article.
-
Isoliquiritigenin inhibits the proliferation of human renal carcinoma Caki cells through the ROS-mediated regulation of the Jak2/STAT3 pathway.Oncol Rep. 2017 Jul;38(1):575-583. doi: 10.3892/or.2017.5677. Epub 2017 May 30. Oncol Rep. 2017. PMID: 28560439
Cited by
-
The Therapeutic and Preventive Efficacy of Curcumin and Its Derivatives in Esophageal Cancer.Asian Pac J Cancer Prev. 2019 May 25;20(5):1329-1337. doi: 10.31557/APJCP.2019.20.5.1329. Asian Pac J Cancer Prev. 2019. PMID: 31127885 Free PMC article. Review.
-
Modulation of aryl hydrocarbon receptor inhibits esophageal squamous cell carcinoma progression by repressing COX2/PGE2/STAT3 axis.J Cell Commun Signal. 2020 Jun;14(2):175-192. doi: 10.1007/s12079-019-00535-5. Epub 2020 Jan 10. J Cell Commun Signal. 2020. PMID: 31925646 Free PMC article.
-
Phlorizin from sweet tea inhibits the progress of esophageal cancer by antagonizing the JAK2/STAT3 signaling pathway.Oncol Rep. 2021 Jul;46(1):137. doi: 10.3892/or.2021.8088. Epub 2021 May 26. Oncol Rep. 2021. PMID: 34036398 Free PMC article.
-
Molecular mechanism, regulation, and therapeutic targeting of the STAT3 signaling pathway in esophageal cancer (Review).Int J Oncol. 2022 Sep;61(3):105. doi: 10.3892/ijo.2022.5395. Epub 2022 Jul 20. Int J Oncol. 2022. PMID: 35856449 Free PMC article.
-
Cancer Chemoprevention: A Strategic Approach Using Phytochemicals.Front Pharmacol. 2022 Jan 13;12:809308. doi: 10.3389/fphar.2021.809308. eCollection 2021. Front Pharmacol. 2022. PMID: 35095521 Free PMC article. Review.
References
-
- Aggarwal B. B., Kumar A., Bharti A. C. (2003). Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 23 363–398. - PubMed
-
- Assi H. H., Paran C., VanderVeen N., Savakus J., Doherty R., Petruzzella E., et al. (2014). Preclinical characterization of signal transducer and activator of transcription 3 small molecule inhibitors for primary and metastatic brain cancer therapy. J. Pharmacol. Exp. Ther. 349 458–469. 10.1124/jpet.114.214619 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

