C. Elegans Fatty Acid Two-Hydroxylase Regulates Intestinal Homeostasis by Affecting Heptadecenoic Acid Production
- PMID: 30184537
- PMCID: PMC6428043
- DOI: 10.1159/000493226
C. Elegans Fatty Acid Two-Hydroxylase Regulates Intestinal Homeostasis by Affecting Heptadecenoic Acid Production
Abstract
Background/aims: The hydroxylation of fatty acids at the C-2 position is the first step of fatty acid α-oxidation and generates sphingolipids containing 2-hydroxy fatty acyl moieties. Fatty acid 2-hydroxylation is catalyzed by Fatty acid 2-hydroxylase (FA2H) enzyme. However, the precise roles of FA2H and fatty acid 2-hydroxylation in whole cell homeostasis still remain unclear.
Methods: Here we utilize Caenorhabditis elegans as the model and systemically investigate the physiological functions of FATH-1/C25A1.5, the highly conserved worm homolog for mammalian FA2H enzyme. Immunostaining, dye-staining and translational fusion reporters were used to visualize FATH-1 protein and a variety of subcellular structures. The "click chemistry" method was employed to label 2-OH fatty acid in vivo. Global and tissue-specific RNAi knockdown experiments were performed to inactivate FATH-1 function. Lipid analysis of the fath-1 deficient mutants was achieved by mass spectrometry.
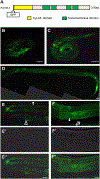
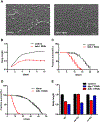
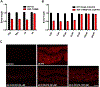
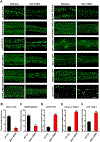
Results: C. elegans FATH-1 is expressed at most developmental stages and in most tissues. Loss of fath-1 expression results in severe growth retardation and shortened lifespan. FATH-1 function is crucially required in the intestine but not the epidermis with stereospecificity. The "click chemistry" labeling technique showed that the FATH-1 metabolites are mainly enriched in membrane structures preferable to the apical side of the intestinal cells. At the subcellular level, we found that loss of fath-1 expression inhibits lipid droplets formation, as well as selectively disrupts peroxisomes and apical endosomes. Lipid analysis of the fath-1 deficient animals revealed a significant reduction in the content of heptadecenoic acid, while other major FAs remain unaffected. Feeding of exogenous heptadecenoic acid (C17: 1), but not oleic acid (C18: 1), rescues the global and subcellular defects of fath-1 knockdown worms.
Conclusion: Our study revealed that FATH-1 and its catalytic products are highly specific in the context of chirality, C-chain length, spatial distribution, as well as the types of cellular organelles they affect. Such an unexpected degree of specificity for the synthesis and functions of hydroxylated FAs helps to regulate protein transport and fat metabolism, therefore maintaining the cellular homeostasis of the intestinal cells. These findings may help our understanding of FA2H functions across species, and offer potential therapeutical targets for treating FA2H-related diseases.
Keywords: 2-hydroxylation; C. elegans; FA2H; Fatty acids; Heptadecenoic acid.
© 2018 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Disclosure Statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures

Similar articles
-
The human FA2H gene encodes a fatty acid 2-hydroxylase.J Biol Chem. 2004 Nov 19;279(47):48562-8. doi: 10.1074/jbc.M406649200. Epub 2004 Aug 27. J Biol Chem. 2004. PMID: 15337768
-
Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation.J Biol Chem. 2007 May 4;282(18):13211-9. doi: 10.1074/jbc.M611562200. Epub 2007 Mar 12. J Biol Chem. 2007. PMID: 17355976 Clinical Trial.
-
FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain.J Lipid Res. 2006 Dec;47(12):2772-80. doi: 10.1194/jlr.M600362-JLR200. Epub 2006 Sep 23. J Lipid Res. 2006. PMID: 16998236
-
Fatty acid 2-Hydroxylation in mammalian sphingolipid biology.Biochim Biophys Acta. 2010 Apr;1801(4):405-14. doi: 10.1016/j.bbalip.2009.12.004. Epub 2009 Dec 21. Biochim Biophys Acta. 2010. PMID: 20026285 Free PMC article. Review.
-
Fatty Acid 2-Hydroxylase and 2-Hydroxylated Sphingolipids: Metabolism and Function in Health and Diseases.Int J Mol Sci. 2023 Mar 3;24(5):4908. doi: 10.3390/ijms24054908. Int J Mol Sci. 2023. PMID: 36902339 Free PMC article. Review.
Cited by
-
Lipid Dyshomeostasis and Inherited Cerebellar Ataxia.Mol Neurobiol. 2022 Jun;59(6):3800-3828. doi: 10.1007/s12035-022-02826-2. Epub 2022 Apr 14. Mol Neurobiol. 2022. PMID: 35420383 Free PMC article. Review.
-
Hydroxylated sphingolipid biosynthesis regulates photoreceptor apical domain morphogenesis.J Cell Biol. 2020 Dec 7;219(12):e201911100. doi: 10.1083/jcb.201911100. J Cell Biol. 2020. PMID: 33048164 Free PMC article.
-
Determination of Genetic Effects of LIPK and LIPJ Genes on Milk Fatty Acids in Dairy Cattle.Genes (Basel). 2019 Jan 28;10(2):86. doi: 10.3390/genes10020086. Genes (Basel). 2019. PMID: 30696079 Free PMC article.
-
Neurodegenerative Disorders: Spotlight on Sphingolipids.Int J Mol Sci. 2021 Nov 5;22(21):11998. doi: 10.3390/ijms222111998. Int J Mol Sci. 2021. PMID: 34769423 Free PMC article. Review.
-
A new model for fatty acid hydroxylase-associated neurodegeneration reveals mitochondrial and autophagy abnormalities.Front Cell Dev Biol. 2022 Dec 14;10:1000553. doi: 10.3389/fcell.2022.1000553. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36589738 Free PMC article.
References
-
- Ibarguren M, Lopez DJ, Escriba PV: The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim Biophys Acta 2014;1838:1518–1528. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
