Identification of the Potential Virulence Factors and RNA Silencing Suppressors of Mulberry Mosaic Dwarf-Associated Geminivirus
- PMID: 30177616
- PMCID: PMC6163789
- DOI: 10.3390/v10090472
Identification of the Potential Virulence Factors and RNA Silencing Suppressors of Mulberry Mosaic Dwarf-Associated Geminivirus
Abstract
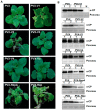
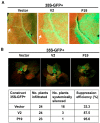
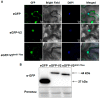
Plant viruses encode virulence factors or RNA silencing suppressors to reprogram plant cellular processes or to fine-tune host RNA silencing-mediated defense responses. In a previous study, Mulberry mosaic dwarf-associated virus (MMDaV), a novel, highly divergent geminivirus, has been identified from a Chinese mulberry tree showing mosaic and dwarfing symptoms, but the functions of its encoded proteins are unknown. In this study, all seven proteins encoded by MMDaV were screened for potential virulence and RNA silencing suppressor activities. We found that V2, RepA, and Rep affect the pathogenicity of a heterologous potato virus X. We showed that V2 could inhibit local RNA silencing and long-distance movement of the RNA silencing signal, but not short-range spread of the green fluorescent protein (GFP) silencing signal in Nicotiana benthamiana 16c plants. In addition, V2 localized to both subnuclear foci and the cytoplasm. Deletion mutagenesis of V2 showed that the basic motif from amino acids 61 to 76 was crucial for V2 to form subnuclear foci and for suppression of RNA silencing. Although the V2 protein encoded by begomoviruses or a curtovirus has been shown to have silencing suppressor activity, this is the first identification of an RNA silencing suppressor from a woody plant-infecting geminivirus.
Keywords: MMDaV; RNA silencing; suppressor; virulence factor.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures


Similar articles
-
Identification and subcellular location of an RNA silencing suppressor encoded by mulberry crinkle leaf virus.Virology. 2019 Jan 2;526:45-51. doi: 10.1016/j.virol.2018.10.007. Epub 2018 Oct 17. Virology. 2019. PMID: 30342301
-
Identification and molecular characterization of a novel monopartite geminivirus associated with mulberry mosaic dwarf disease.J Gen Virol. 2015 Aug;96(8):2421-2434. doi: 10.1099/vir.0.000175. Epub 2015 May 6. J Gen Virol. 2015. PMID: 25953916
-
Replication-associated proteins encoded by Wheat dwarf virus act as RNA silencing suppressors.Virus Res. 2014 Sep 22;190:34-9. doi: 10.1016/j.virusres.2014.06.014. Epub 2014 Jul 9. Virus Res. 2014. PMID: 25016035
-
Silencing suppression by geminivirus proteins.Virology. 2006 Jan 5;344(1):158-68. doi: 10.1016/j.virol.2005.09.041. Virology. 2006. PMID: 16364747 Review.
-
Viral suppressors of RNA silencing.Trends Plant Sci. 2011 May;16(5):265-72. doi: 10.1016/j.tplants.2011.02.010. Epub 2011 Mar 24. Trends Plant Sci. 2011. PMID: 21439890 Review.
Cited by
-
Two genes encoded by mulberry crinkle leaf virus (MCLV): The V4 gene enhances viral replication, and the V5 gene is needed for MCLV infection in Nicotiana benthamiana.Virus Res. 2024 Jan 2;339:199288. doi: 10.1016/j.virusres.2023.199288. Epub 2023 Dec 6. Virus Res. 2024. PMID: 38043724 Free PMC article.
-
Functional Analysis of V2 Protein of Beet Curly Top Iran Virus.Plants (Basel). 2022 Dec 2;11(23):3351. doi: 10.3390/plants11233351. Plants (Basel). 2022. PMID: 36501393 Free PMC article.
-
Identification and Functional Analysis of Four RNA Silencing Suppressors in Begomovirus Croton Yellow Vein Mosaic Virus.Front Plant Sci. 2022 Jan 7;12:768800. doi: 10.3389/fpls.2021.768800. eCollection 2021. Front Plant Sci. 2022. PMID: 35069624 Free PMC article.
-
Identification of RNA silencing suppressor encoded by citrus chlorotic dwarf-associated virus.Front Microbiol. 2024 Jan 25;15:1328289. doi: 10.3389/fmicb.2024.1328289. eCollection 2024. Front Microbiol. 2024. PMID: 38333582 Free PMC article.
-
RepA Promotes the Nucleolar Exclusion of the V2 Protein of Mulberry Mosaic Dwarf-Associated Virus.Front Microbiol. 2020 Aug 4;11:1828. doi: 10.3389/fmicb.2020.01828. eCollection 2020. Front Microbiol. 2020. PMID: 32903838 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

