Alleviation of endoplasmic reticulum stress protects against cisplatin-induced ovarian damage
- PMID: 30176887
- PMCID: PMC6122480
- DOI: 10.1186/s12958-018-0404-4
Alleviation of endoplasmic reticulum stress protects against cisplatin-induced ovarian damage
Abstract
Background: Cisplatin (CDDP), a widely used chemotherapeutic agent, can induce excessive granulosa cell apoptosis, follicle loss and even premature ovarian insufficiency (POI). However, the mechanism remains elusive, although some studies have indicated the involvement of endoplasmic reticulum stress (ERS). The aim of our study was to investigate the possible mechanism ERS in CDDP-induced granulosa cell apoptosis and follicle loss.
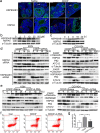
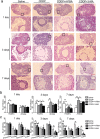
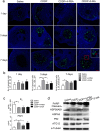
Methods: A POI mouse model was generated by CDDP. The ovaries samples were collected and processed for isobaric tags for relative and absolute quantification analysis (iTRAQ) to screen out our interested proteins of HSPA5 and HSP90AB1, and the decline in their expression were verified by a real-time quantitative PCR and a western blotting assay. In vitro, human granulosa cells, KGN and COV434 cells were transfected with siRNA targeting HSPA5 and HSP90AB1 and then treated with CDDP, or treated with CDDP with/without CDDP+ 4-phenylbutyric acid (4-PBA) and 3-methyladenine (3-MA). The levels of ERS, autophagy and apoptosis were evaluated by western blotting, DALGreen staining and flow cytometry. In vivo, ovaries from mice that received intraperitoneal injections of saline, CDDP, CDDP+ 4-PBA and CDDP+ 3-MA were assayed by immunofluorescence, hematoxylin and eosin (H&E) staining for follicle counting, and terminal-deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining for cell apoptosis assay. The plasma hormone levels were measured by an enzyme-linked immunosorbent assay (ELISA) kit.
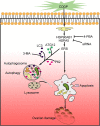
Results: We have clarified the relationships between ERS, autophagy, and apoptosis in CDDP-induced granulosa cell apoptosis, both in vitro and in vivo. Alleviating ERS by inhibiting HSPA5 and HSP90AB1 attenuated CDDP-induced autophagy and apoptosis. 4-PBA treatment significantly attenuated CDDP-induced cell autophagy and apoptosis in cultured KGN and COV434 cells. However, inhibiting cell autophagy with 3-MA negligibly restored the CDDP-induced changes in ERS and apoptosis. In vivo experiments also demonstrated that treatment with 4-PBA, but not 3-MA, prevented CDDP-induced ovarian damage and hormone dysregulation.
Conclusions: CDDP-induced ERS could promote autophagy and apoptosis in granulosa cells, causing excessive follicle loss and endocrine disorders. Alleviation of ERS with 4-PBA, but not of autophagy with 3-MA, protect against CDDP-induced granulosa cell apoptosis and ovarian damage. Thus, 4-PBA can be used to protect the ovary during chemotherapy in women.
Keywords: 4-PBA; Cisplatin; Endoplasmic reticulum stress; Granulosa cell apoptosis; Ovarian damage.
Conflict of interest statement
Ethics approval
All animal experiments were approved by the Southern Medical University Committee on the Use and Care of Animals and were performed in accordance with the Committee’s guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
The mechanism of curcumin to protect mouse ovaries from oxidative damage by regulating AMPK/mTOR mediated autophagy.Phytomedicine. 2024 Jun;128:155468. doi: 10.1016/j.phymed.2024.155468. Epub 2024 Feb 24. Phytomedicine. 2024. PMID: 38471315
-
Ubiquitin-like modifier 1 ligating enzyme 1 relieves cisplatin-induced premature ovarian failure by reducing endoplasmic reticulum stress in granulosa cells.Reprod Biol Endocrinol. 2022 May 24;20(1):84. doi: 10.1186/s12958-022-00956-9. Reprod Biol Endocrinol. 2022. PMID: 35610622 Free PMC article.
-
Endoplasmic reticulum stress promotes autophagy and apoptosis and reverses chemoresistance in human ovarian cancer cells.Oncotarget. 2017 Jul 25;8(30):49380-49394. doi: 10.18632/oncotarget.17673. Oncotarget. 2017. PMID: 28537902 Free PMC article.
-
The Role of Heat Shock Proteins in Cisplatin Resistance.Anticancer Agents Med Chem. 2018;18(15):2093-2109. doi: 10.2174/1871520618666180817114952. Anticancer Agents Med Chem. 2018. PMID: 30156165 Review.
-
SIRT1 regulates endoplasmic reticulum stress-related organ damage.Acta Histochem. 2024 Jan;126(1):152134. doi: 10.1016/j.acthis.2024.152134. Epub 2024 Jan 17. Acta Histochem. 2024. PMID: 38237370 Review.
Cited by
-
hUMSC transplantation restores ovarian function in POI rats by inhibiting autophagy of theca-interstitial cells via the AMPK/mTOR signaling pathway.Stem Cell Res Ther. 2020 Jul 3;11(1):268. doi: 10.1186/s13287-020-01784-7. Stem Cell Res Ther. 2020. PMID: 32620136 Free PMC article.
-
[miR-483-5p aggravates cisplatin-induced premature ovarian insufficiency in rats by targeting FKBP4].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Jun 20;41(6):801-810. doi: 10.12122/j.issn.1673-4254.2021.06.01. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34238731 Free PMC article. Chinese.
-
Comprehensive analysis of lncRNA-miRNA-mRNA ceRNA network and key genes in granulosa cells of patients with biochemical primary ovarian insufficiency.J Assist Reprod Genet. 2024 Jan;41(1):15-29. doi: 10.1007/s10815-023-02937-2. Epub 2023 Oct 17. J Assist Reprod Genet. 2024. PMID: 37847421 Free PMC article.
-
Revealing the Mechanism of Friedelin in the Treatment of Ulcerative Colitis Based on Network Pharmacology and Experimental Verification.Evid Based Complement Alternat Med. 2021 Nov 2;2021:4451779. doi: 10.1155/2021/4451779. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34765000 Free PMC article.
-
Investigating the potential role of α-SNAP in preventing chemotherapy-induced ovarian dysfunction: Insights from cellular and animal models.Heliyon. 2024 Jun 10;10(12):e32802. doi: 10.1016/j.heliyon.2024.e32802. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38994045 Free PMC article.
References
-
- European Society for Human R, embryology guideline group on POI. Webber L, Davies M, Anderson R, Bartlett J, Braat D, cartwright B, Cifkova R, de Muinck Keizer-Schrama S, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–937. doi: 10.1093/humrep/dew027. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

