Copanlisib for treatment of B-cell malignancies: the development of a PI3K inhibitor with considerable differences to idelalisib
- PMID: 30174412
- PMCID: PMC6109662
- DOI: 10.2147/DDDT.S142406
Copanlisib for treatment of B-cell malignancies: the development of a PI3K inhibitor with considerable differences to idelalisib
Abstract
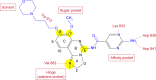
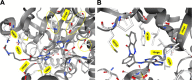
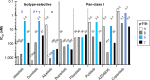
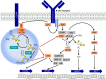
On the occasion of its recent approval for relapsed follicular lymphoma, we review the design and development of the pan-class I PI3K inhibitor copanlisib as a drug for the treatment of B-cell malignancies in comparison with other kinase inhibitors targeting B-cell-receptor signaling, in particular with strictly isoform-δ-selective idelalisib. In agreement with previously defined PI3K-inhibitor chemotypes, the 2,3-dihydroimidazo[1,2-c]quinazoline scaffold of copanlisib adopts a flat conformation in the adenine-binding pocket of the catalytic p110 subunit and further extends into a deeper-affinity pocket in contrast to idelalisib, the quinazoline moiety of which is accommodated in a newly created selectivity pocket. Copanlisib shows higher potency than other clinically developed PI3K inhibitors against all four class I isoforms, with approximately tenfold preference for p110α and p110δ. Owing to its potency and isoform profile, copanlisib exhibits cell-type-specific cytotoxicity against primary chronic lymphocytic leukemia cells and diffuse large B-cell lymphoma (DLBCL) cell lines at nanomolar concentrations. Moreover, copanlisib differs from idelalisib in regard to intravenous versus oral administration and weekly versus twice-daily dosing. In regard to adverse effects, intermittent intravenous treatment with copanlisib leads to fewer gastrointestinal toxicities compared with continuous oral dosing of idelalisib. In relapsed follicular lymphoma, copanlisib appears more effective and especially better tolerated than other targeted therapies. Copanlisib extends existing treatment options for this subtype of indolent non-Hodgkin lymphoma and also shows promising response rates in DLBCL, especially of the activated B-cell type.
Keywords: B-cell receptor signaling; leukemia; non-Hodgkin lymphoma; p110 isoforms; targeted therapy.
Conflict of interest statement
Disclosure GK has received research funding from Bayer, Roche, and Boehringer Ingelheim. MH has received consultancy fees and honoraria from AbbVie, Mundipharma, GlaxoSmithKline, Gilead, and Celgene; consultancy, honoraria, and speakers bureau fees from Janssen; consultancy and speakers bureau fees from Pharmacyclics; consultancy, research funding, and speakers bureau fees from Roche; and funding from Gilead. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Efficacy of phosphatidylinositol-3 kinase inhibitors with diverse isoform selectivity profiles for inhibiting the survival of chronic lymphocytic leukemia cells.Int J Cancer. 2015 Nov 1;137(9):2234-42. doi: 10.1002/ijc.29579. Epub 2015 May 12. Int J Cancer. 2015. PMID: 25912635
-
PI3Kδ-selective and PI3Kα/δ-combinatorial inhibitors in clinical development for B-cell non-Hodgkin lymphoma.Expert Opin Investig Drugs. 2017 Nov;26(11):1267-1279. doi: 10.1080/13543784.2017.1384815. Epub 2017 Oct 6. Expert Opin Investig Drugs. 2017. PMID: 28945111 Free PMC article. Review.
-
Idelalisib.Recent Results Cancer Res. 2018;212:243-264. doi: 10.1007/978-3-319-91439-8_12. Recent Results Cancer Res. 2018. PMID: 30069634 Review.
-
Properties of FDA-approved small molecule phosphatidylinositol 3-kinase inhibitors prescribed for the treatment of malignancies.Pharmacol Res. 2021 Jun;168:105579. doi: 10.1016/j.phrs.2021.105579. Epub 2021 Mar 26. Pharmacol Res. 2021. PMID: 33774181 Review.
-
The PI3K p110δ Isoform Inhibitor Idelalisib Preferentially Inhibits Human Regulatory T Cell Function.J Immunol. 2019 Mar 1;202(5):1397-1405. doi: 10.4049/jimmunol.1701703. Epub 2019 Jan 28. J Immunol. 2019. PMID: 30692213
Cited by
-
New prospectives on treatment opportunities in RASopathies.Am J Med Genet C Semin Med Genet. 2022 Dec;190(4):541-560. doi: 10.1002/ajmg.c.32024. Epub 2022 Dec 19. Am J Med Genet C Semin Med Genet. 2022. PMID: 36533679 Free PMC article. Review.
-
PI3Kδ Pathway Dysregulation and Unique Features of Its Inhibition by Leniolisib in Activated PI3Kδ Syndrome and Beyond.J Allergy Clin Immunol Pract. 2024 Jan;12(1):69-78. doi: 10.1016/j.jaip.2023.09.016. Epub 2023 Sep 28. J Allergy Clin Immunol Pract. 2024. PMID: 37777067 Free PMC article.
-
ETV6/RUNX1 Fusion Gene Abrogation Decreases the Oncogenicity of Tumour Cells in a Preclinical Model of Acute Lymphoblastic Leukaemia.Cells. 2020 Jan 15;9(1):215. doi: 10.3390/cells9010215. Cells. 2020. PMID: 31952221 Free PMC article.
-
Copanlisib in the Treatment of Relapsed Follicular Lymphoma: Utility and Experience from the Clinic.Cancer Manag Res. 2021 Jan 25;13:677-692. doi: 10.2147/CMAR.S201024. eCollection 2021. Cancer Manag Res. 2021. PMID: 33531838 Free PMC article. Review.
-
Copanlisib promotes growth inhibition and apoptosis by modulating the AKT/FoxO3a/PUMA axis in colorectal cancer.Cell Death Dis. 2020 Nov 2;11(11):943. doi: 10.1038/s41419-020-03154-w. Cell Death Dis. 2020. PMID: 33139695 Free PMC article.
References
-
- Hallek M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2017;92(9):946–965. - PubMed
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. - PubMed
-
- Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

