Cinnamic aldehyde inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in Zucker Diabetic Fatty rats
- PMID: 30172101
- PMCID: PMC6122148
- DOI: 10.1016/j.redox.2018.08.013
Cinnamic aldehyde inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in Zucker Diabetic Fatty rats
Abstract
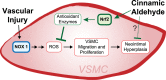
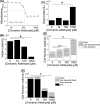
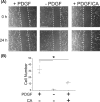
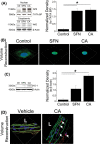
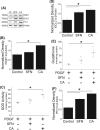
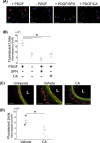
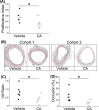
Atherosclerosis remains the number one cause of death and disability worldwide. Atherosclerosis is treated by revascularization procedures to restore blood flow to distal tissue, but these procedures often fail due to restenosis secondary to neointimal hyperplasia. Diabetes mellitus is a metabolic disorder that accelerates both atherosclerosis development and onset of restenosis. Strategies to inhibit restenosis aim at reducing neointimal hyperplasia by inhibiting vascular smooth muscle cell (VSMC) proliferation and migration. Since increased production of reactive oxygen species promotes VSMC proliferation and migration, redox intervention to maintain vascular wall redox homeostasis holds the potential to inhibit arterial restenosis. Cinnamic aldehyde (CA) is an electrophilic Nrf2 activator that has shown therapeutic promise in diabetic rodent models. Nrf2 is a transcription factor that regulates the antioxidant response. Therefore, we hypothesized that CA would activate Nrf2 and would inhibit neointimal hyperplasia after carotid artery balloon injury in the Zucker Diabetic Fatty (ZDF) rat. In primary ZDF VSMC, CA inhibited cell growth by MTT with an EC50 of 118 ± 7 μM. At a therapeutic dose of 100 μM, CA inhibited proliferation of ZDF VSMC in vitro and reduced the proliferative index within the injured artery in vivo, as well as migration of ZDF VSMC in vitro. CA activated the Nrf2 pathway in both ZDF VSMC and injured carotid arteries while also increasing antioxidant defenses and reducing markers of redox dysfunction. Additionally, we noted a significant reduction of neutrophils (69%) and macrophages (78%) within the injured carotid arteries after CA treatment. Lastly, CA inhibited neointimal hyperplasia evidenced by a 53% reduction in the intima:media ratio and a 61% reduction in vessel occlusion compared to arteries treated with vehicle alone. Overall CA was capable of activating Nrf2, and inhibiting neointimal hyperplasia after balloon injury in a rat model of diabetic restenosis.
Keywords: Cinnamic aldehyde; Diabetes; Neointimal hyperplasia; Nrf2; Restenosis; Vascular smooth muscle cells.
Copyright © 2018. Published by Elsevier B.V.
Figures


Similar articles
-
Nitric oxide differentially affects proteasome activator 28 after arterial injury in type 1 and type 2 diabetic rats.J Surg Res. 2016 May 15;202(2):413-21. doi: 10.1016/j.jss.2016.01.030. Epub 2016 Jan 30. J Surg Res. 2016. PMID: 27229117 Free PMC article.
-
N-oleoylethanolamide suppresses intimal hyperplasia after balloon injury in rats through AMPK/PPARα pathway.Biochem Biophys Res Commun. 2018 Feb 5;496(2):415-421. doi: 10.1016/j.bbrc.2018.01.015. Epub 2018 Jan 4. Biochem Biophys Res Commun. 2018. PMID: 29305859
-
Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of UbcH10.Cell Biochem Biophys. 2011 Jun;60(1-2):89-97. doi: 10.1007/s12013-011-9179-3. Cell Biochem Biophys. 2011. PMID: 21448667 Free PMC article.
-
Noncoding RNAs in vascular smooth muscle cell function and neointimal hyperplasia.FEBS J. 2020 Dec;287(24):5260-5283. doi: 10.1111/febs.15357. Epub 2020 May 22. FEBS J. 2020. PMID: 32367680 Review.
-
Intimal hyperplasia in murine models.Curr Drug Targets. 2008 Mar;9(3):251-60. doi: 10.2174/138945008783755601. Curr Drug Targets. 2008. PMID: 18336244 Free PMC article. Review.
Cited by
-
Down-regulation of Suv39h1 attenuates neointima formation after carotid artery injury in diabetic rats.J Cell Mol Med. 2020 Jan;24(1):973-983. doi: 10.1111/jcmm.14809. Epub 2019 Nov 17. J Cell Mol Med. 2020. PMID: 31736204 Free PMC article.
-
Nrf2-Mediated Dichotomy in the Vascular System: Mechanistic and Therapeutic Perspective.Cells. 2022 Sep 28;11(19):3042. doi: 10.3390/cells11193042. Cells. 2022. PMID: 36231004 Free PMC article. Review.
-
Delivery of Cinnamic Aldehyde Antioxidant Response Activating nanoParticles (ARAPas) for Vascular Applications.Antioxidants (Basel). 2021 Apr 29;10(5):709. doi: 10.3390/antiox10050709. Antioxidants (Basel). 2021. PMID: 33946889 Free PMC article.
-
A Rat Carotid Artery Pressure-Controlled Segmental Balloon Injury with Periadventitial Therapeutic Application.J Vis Exp. 2020 Jul 9;(161):10.3791/60473. doi: 10.3791/60473. J Vis Exp. 2020. PMID: 32716387 Free PMC article.
-
The Therapeutic Roles of Cinnamaldehyde against Cardiovascular Diseases.Oxid Med Cell Longev. 2022 Oct 8;2022:9177108. doi: 10.1155/2022/9177108. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2024 Jan 9;2024:9815890. doi: 10.1155/2024/9815890. PMID: 36254234 Free PMC article. Retracted. Review.
References
-
- Writing Group M., Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J. Executive summary: heart disease and stroke statistics–2016 update: a report from the American heart association. Circulation. 2016;133:447–454. - PubMed
-
- International Diabetes Federation Diabetes Atlas, Eighth edition, 2017.
-
- DeRubertis B.G., Pierce M., Ryer E.J., Trocciola S., Kent K.C., Faries P.L. Reduced primary patency rate in diabetic patients after percutaneous intervention results from more frequent presentation with limb-threatening ischemia. J. Vasc. Surg. 2008;47:101–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

