LFA-1 Ligation by High-Density ICAM-1 Is Sufficient To Activate IFN-γ Release by Innate T Lymphocytes
- PMID: 30171164
- PMCID: PMC6179935
- DOI: 10.4049/jimmunol.1800537
LFA-1 Ligation by High-Density ICAM-1 Is Sufficient To Activate IFN-γ Release by Innate T Lymphocytes
Abstract
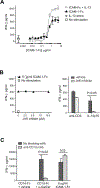
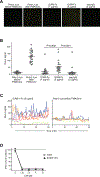
By binding to its ligand ICAM-1, LFA-1 is known to mediate both adhesion and costimulatory signaling for T cell activation. The constitutively high LFA-1 cell surface expression of invariant NKT (iNKT) cells has been shown to be responsible for their distinctive tissue homing and residency within ICAM-rich endothelial vessels. However, the functional impact of LFA-1 on the activation of iNKT cells and other innate T lymphocyte subsets has remained largely unexplored. In particular, it is not clear whether LFA-1 contributes to innate-like pathways of T cell activation, such as IFN-γ secretion in response to IL-12. Using a recombinant ICAM-1-Fc fusion protein to stimulate human iNKT cells in the absence of APCs, we show that LFA-1 engagement enhances their IL-12-driven IFN-γ production. Surprisingly, exposure to high densities of ICAM-1 was also sufficient to activate iNKT cell cytokine secretion independently of IL-12 and associated JAK/STAT signaling. LFA-1 engagement induced elevated cytoplasmic Ca2+ and rapid ERK phosphorylation in iNKT cells, and the resulting IFN-γ secretion was dependent on both of these pathways. Analysis of freshly isolated human PBMC samples revealed that a fraction of lymphocytes that showed elevated LFA-1 cell surface expression produced IFN-γ in response to plate-bound ICAM-1-Fc. A majority of the responding cells were T cells, with the remainder NK cells. The responding T cells included iNKT cells, MAIT cells, and Vδ2+ γδ T cells. These results delineate a novel integrin-mediated pathway of IFN-γ secretion that is a shared feature of innate lymphocytes.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures




Similar articles
-
Co-engagement of CD3 with LFA-1 or ICAM-1 adhesion molecules enhances the frequency of activation of single murine CD4+ and CD8+ T cells and induces synthesis of IL-3 and IFN-gamma but not IL-4 or IL-6.Int Immunol. 1992 Apr;4(4):475-85. doi: 10.1093/intimm/4.4.475. Int Immunol. 1992. PMID: 1350461
-
The proliferative response of human T cells to allogeneic IFN-gamma-treated endothelial cells is mediated via both CD2/LFA-3 and LFA-1/ICAM-1 and -2 adhesion pathways.Transpl Immunol. 1993;1(3):183-91. doi: 10.1016/0966-3274(93)90045-a. Transpl Immunol. 1993. PMID: 7521739
-
Sedative drug modulates T-cell and lymphocyte function-associated antigen-1 function.Anesth Analg. 2011 Apr;112(4):830-8. doi: 10.1213/ANE.0b013e31820dcabb. Epub 2011 Mar 8. Anesth Analg. 2011. PMID: 21385989 Free PMC article.
-
T Cell Activation Pathways: B7, LFA-3, and ICAM-1 Shape Unique T Cell Profiles.Crit Rev Immunol. 2017;37(2-6):463-481. doi: 10.1615/CritRevImmunol.v37.i2-6.130. Crit Rev Immunol. 2017. PMID: 29773030 Review.
-
T cell activation pathways: B7, LFA-3, and ICAM-1 shape unique T cell profiles.Crit Rev Immunol. 1995;15(3-4):235-53. doi: 10.1615/critrevimmunol.v15.i3-4.30. Crit Rev Immunol. 1995. PMID: 8834450 Review.
Cited by
-
Motility and tumor infiltration are key aspects of invariant natural killer T cell anti-tumor function.Nat Commun. 2024 Feb 9;15(1):1213. doi: 10.1038/s41467-024-45208-z. Nat Commun. 2024. PMID: 38332012 Free PMC article.
-
Human CD4+ iNKT cell adoptive immunotherapy induces anti-tumour responses against CD1d-negative EBV-driven B lymphoma.Immunology. 2024 Aug;172(4):627-640. doi: 10.1111/imm.13799. Epub 2024 May 13. Immunology. 2024. PMID: 38736328
-
Vγ9Vδ2 T Cells Activation Through Phosphoantigens Can Be Impaired by a RHOB Rerouting in Lung Cancer.Front Immunol. 2020 Jul 7;11:1396. doi: 10.3389/fimmu.2020.01396. eCollection 2020. Front Immunol. 2020. PMID: 32733462 Free PMC article.
-
Thymic development of human natural killer T cells: recent advances and implications for immunotherapy.Front Immunol. 2024 Aug 29;15:1441634. doi: 10.3389/fimmu.2024.1441634. eCollection 2024. Front Immunol. 2024. PMID: 39267746 Free PMC article. Review.
-
Diminished expression of major histocompatibility complex facilitates the use of human induced pluripotent stem cells in monkey.Stem Cell Res Ther. 2020 Aug 3;11(1):334. doi: 10.1186/s13287-020-01847-9. Stem Cell Res Ther. 2020. PMID: 32746912 Free PMC article.
References
-
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, and Sant’Angelo DB 2008. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol 9: 1055–1064. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

